PHSI3006 PHYSIOLOGY 3006/3906 1) A) Define hormone
advertisement

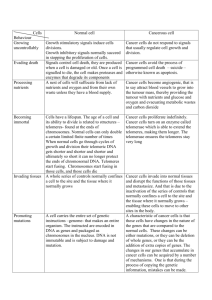
PHSI3006 PHYSIOLOGY 3006/3906 1) A) Define hormone resistance Hormone resistance is a condition in which normal concentrations of a hormone fail to produce a normal level of response (in cells, tissues or the whole organism). B) In Vitamin D Resistance, describe two clinical symptoms and the mechanism Individuals with Vitamin D Resistance may develop a number of symptoms such as hypocalcaemia, secondary hyperparathyroidism, rickets, and impaired bone mineralisation. - Hypocalcaemia: normally, interaction of 1,25(OH)2D with the VDR increases the efficiency of Ca2+ and phosphate absorption in the gut but since the organ is resistant to vitamin D then 1,25(OH)2D would not be able to bind to the VDR properly, hence calcium absorption is decreased leading to hypocalcaemia. - Secondary hyperparathyroidism: during VD resistance, 1,25(OH)2D is not producing a normal response, resulting in decreased calcium absorption in the gut which is then sensed by the parathyroid gland to release PTH. PTH then stimulates the kidney to increase 1,25(OH)2D production. C) Are there similar resistance disease for other steroid receptors, and describe one. Yes, there is also androgen resistance. Androgen is a steroid hormone and its receptor is part of the nuclear receptor steroid family, like vitamin D. It is activated by binding of androgen hormones (testosterone) in the cytoplasm and translocating in the nucleus. The main function of the androgen receptor is a DNA-binding transcription factor that regulates gene expression. However, the gene expression of the androgen receptor on the X chromosome can be interrupted by a variety of mutations and cause a variety of syndromes from complete testicular feminisation in men to some degree of infertility. 2) Graph with thymine dimerisations i) Which axis represents DNA damage? y-axis ii) What can be said about Vitamin D on the number of dimers Reduces it iii) Describe effect of Vitamin D antagonists on thymine dimers No effect by themselves, level of DNA damage remains high. iv) What happens if you administer the antagonists with Vitamin D? When HL is added with vitamin D, there is no reduction in DNA damage. However, when TE987 is added, there is a reduction in damage. v) What can you say about the mechanism of action of Vitamin D in preventing the dimerisation [~ TE987 & HL, one of the antagonists will reduce vitamin D-mediated dimerisation and the other one has no effect, one of them is classical pathway antagonist and other antagonist is the rapid pathway = thereby vitamin D acts via rapid pathway] The antagonist of the genomic pathway (TEI-9647) has no effect on Vitamin D protecting from DNA damage. However, the antagonist of the non-genomic pathway abolished the effect of vitamin D in protecting from DNA damage. Thus it can be deduced that the action of vitamin D in preventing dimerisation is via the non-genomic pathway. 3) Draw a diagram showing how PTH regulates the paracrine signalling to regulate bone turnover [refer to interactions RANK: RANKL & how osteoclasts are activated] 4) a. Draw the current vs. voltage graph for potassium channels for a whole cell patch clamp. [Nernst potential of -20mV] b. explain the assumptions used to draw the graph 1- The potassium channel is an inwardly rectifying channel, thus the conductance into the cell is greater than the conductance out of the cell. Consequently, the slope in the inward direction is greater than that of the outward direction. 2c. explain what effect potassium channel blockers would have on the reversal potential [refer to I-V graph & Nernst potential concepts] The IV graph shifts to the left. When the potassium channels are blocked, K+ will no longer be able to move into the cell and thus there will be a large resistance 5) a. Graphs on quantal release/amount. How would go about testing this using physiological experiment. Define parameters & how would you investigate. b. explain relationship between frequency & quantal content. i think this is going to be like.. testing using an electrode n that muscle fibre bullshit. 3005 lectures =] 6) Go through the steps of indirect immunofluorescence (flow chart) in detecting autoantibodies at the endplate. must specify the source of the antibodies (both primary & secondary antibodies, mention the control) Secondary, or indirect, immunofluorescence uses two antibodies; the first (the primary antibody) recognises the target molecule and binds to it, and the second (the secondary antibody), which carries the fluorophore, recognises the primary antibody and binds to it. AChRs are found on the post synaptic membrane (endplate). Autoantibodies will bind to these receptors. Anti-AChR antibodies (IgG) bind to these receptors leading to myasthenia gravis. In order, to detect the location of these antibodies when binding to the receptors, indirect imunofluoroscense is used. MUSK is a protein required for the formation of the NMJ. These proteins are gathered on the endplate to form the NMJ. Anti MuSK antibodies bind to MUSK leading to myasthenia gravis A second category of gravis is due to autoantibodies against the MuSK protein (muscle specific kinase), a tyrosine kinase receptor which is required for the formation of the neuromuscular junction. Antibodies against MuSK inhibit the signalling of MuSK normally induced by its nerve-derived ligand, agrin. The result is a decrease in patency of the neuromuscular junction, and the consequent symptoms of MG. How to detect auto-antibodies at the endplate: 1- Incubate cells with affinity purified antibody (IgG) against MUSK proteins. 2- Wash off unbound primary antibody with phosphate buffered saline (PBS) 3- Incubate cells with secondary antibody (anti MUSK IgG) which is chemically conjugated to a fluorescent dye, which binds to the constant regions of the primary antibody. 4- Wash off unbound antibodies with PBS 5- Examine cells with fluorescence microscope Sometimes immunostaining may fail for technical reasons leading to negative results. To be sure this is not a problem, a positive control should be incorporated. 7) a. General KO studies are associated with embryonic lethality. describe some conditional constructs to overcome these. A conditional knockout allows gene deletion in a tissue or time specific manner. This is done by creating a transgenic mouse with short sequence called Lox P on both sides of the gene of interest. This mouse is then crossed with a mouse containing Cre-recombinase which is a viral enzyme that can recognize these sequences. The Cre is then going to cut at the Lox P sites, and that piece of DNA is then going to be excised and LoxP sites recombine. This gives us a mouse with the gene of interest knocked out. b. Explain briefly how you would create a transgenic mouse for Vitamin D KO in hair specific cells of mice. Get a hair cell specific gene only expressed in the hair follicle, use that to drive Cre. Create a transgenic mouse with LoxP on either side of the vitamin D receptor gene of a hair follicle. This mouse is then to be crossed with a mouse containing Cre recombinase which is driven by promoter. Cre will cut at the LoxP sites removing the gene of interest. Lox P sites will recombine and you end up with a specific VDR deletion in the hair follicle. Get a hair cell specific gene only expressed in hair follicle and use that to drive Cre. Hence, you get a mouse that has Cre driven by vitamin D receptor promoter. Cross this mouse with a vitamin D receptor floxed line. Cre recombinase will cut at the lox P sites removing the gene of interest. Lox P sites will recombine and you end up with a specific VDR deletion in the hair follicle and thus we end up with a VDR knockout mouse at the level of hair follicle. 8) What are the 6 hallmarks of cancer? - evading apoptosis - self-sufficiency in growth signals - insensitivity to anti-growth signals - tissue invasion and metastasis - sustained angiogenesis - limitless replicative potential 9) Forgot what it was exactly but i think it had something to do with the 3rd PBL on prostate cancer i think PHSI 3005 exam 1) Explain what an antagonist is and how an antagonist for calcium receptor at PT gland acts to affect bone turnover. Why is an antagonist used over intermittent PTH? An antagonist is a type of receptor ligand or drug that does not provoke a biological response itself upon binding to a receptor, but blocks other agonist mediated responses. An antagonist blocks a receptor from activation by their agonists. Antagonists for calcium receptors act on calcium receptors on the surface of parathyroid glands stimulating the release of the body's own stores of parathyroid hormone (PTH). As a result, PTH thus increases osteoblasts numbers and life span (deceased osteoblasts apoptosis) leading to increased bone mass and connectivity and the decrease of bone fracture and osteoporosis. Antagonists are used over intermittent PTH because intermittent PTH requires daily injection and also because PTH cannot be given for more than 2 years which means it is not a lifestyle solution. 2) Explain why older women are more prone to fractures than men As you get older; bone mass decreases. Women go through menopause causing accelerated bone loss due to the rapid decrease in estrogen. Estrogens in bone promote coupling and there’s some evidence of increased bone formation. Normal estrogen leads to: - Reduced bone resorption via increased osteoprotegerin and via shorter osteoclasts lifespan. (if osteoclasts live longer they can eat away more bone) - better coupling: increased osteoblastic activity - Enhances growth factor incorporation into the bone matrix. A lack of estrogen leads to : - An increase in bone resorption. It is associated with an increase in RANK: OPG ratio. - Impaired coupling: the rate of resorption is much greater than the rate of bone formation. - Bone cells become more susceptible to being killed by oxidative stress Osteocytes are also affected leading to higher sclerostin. High levels of sclerostin inhibit the WNT pathway, inhibiting bone formation. Male sex steroids (testosterone), on the other hand, put bone on the outside of bone, giving them wider bones which are more resistant to bending All these factors make older women more susceptible to fractures. 2) Explain two methods in which auto-antibodies to AChR act to affect the Neuromuscular junction. How is the neuromuscular transmission affected by these effects? Anti-AchR antibodies bind to the main immunogenic region on the alpha-subunit of the AChR. There are three main mechanisms in which auto-immune antibodies to AChRs act to affect the NMJ: 1. Anti AChR antibodies can accelerate AChR degradation by cross-linking adjacent AChRs on the NMJ postsynaptic membrane, facilitating endocytosis of the cross-linked AChR molecules and their phagocytic degradation (antigenic modulation). This ultimately leads to a reduced number of AChR molecules on the postsynaptic membrane and thus the failure of neuromuscular transmission due to the altered binding of ACh to receptors. 2. The second mechanism is that anti-AChRs can initiate complement cascade pathway and the membrane attack complex initiating damage to the postsynaptic membrane. This results in the formation of the membrane attack complex which leads to the destruction of the morphology of the muscle membrane (postsynaptic membrane). This ultimately leads to a simplified, altered morphology of the postsynaptic membrane of the NMJ, which lacks the normal deep junctional folds and has a relatively flat surface. This increases the diffusion of Ach away from the synaptic cleft and reduces the probability of ACh interacting with functional AChR. 3) What is pseudohypoaldosteronism type I? Explain how the phenotype arises and how the symptoms are explained Pseudohypoaldosteronism type 1 is a disorder of electrolyte metabolism characterized by an apparent state of renal tubular unresponsiveness or resistance to the action of aldosterone. This condition is characterized by hyperkalemia, hyponatremia, metabolic acidosis, renal salt wasting, hypertension, despite normal or elevated levels of renin and aldosterone. Pseudohypoaldosteronism type 1 has 2 different forms: a- Autosomal recessive: in which multiple organs are affected such as kidneys, colon, salivary ducts. The phenotype arises due to mutations in α, β, or γ subunits of ENaC which thus leads to the loss of its function and hence the incapability of reabsorbing sodium leading to hyponatemia and consequently to hyprkalemia. b- Autosomal dominant: This form only affects kidneys due to a mutation in MR- this form is not related to ENaC channels. 4) Write brief notes on the SLC26A family SLC26a Family in Gut and Kidney - SLC26 isoforms are members of a large family of anion exchangers. - The majority of these isoforms function as anion exchangers - Modes of transport mediated by SLC26 members include the exchange of chloride for bicarbonate - There are 10 members in the SLC26A family but only 5 transport bicarbonate ions. One example is SLC26A3. - It is found on the apical side of surface and crypt cells of the colon. - It transports salt in collaboration with Na+H+ exchanger. - Recessive loss of function mutations lead to severe congenital chloride diarrhoea causing weight loss - A rare recessive autosomal condition - Mutations result in no expression of SLC26A3 by gut epithelial cells Another member is SCL26A4 - It is expressed in the cochlea, kidney and thyroid - Slc26a4 (pendrin) deletion impairs the ability of the kidney to retain salt during saltdeprivation states - Mutation in this can lead to deafness: - pendred syndrome and enlarged vestibular aqueduct syndrome (EVAS) - Goitre but no renal disease. It is a bicarbonate secretor but can also transport iodine. 5) Write brief notes on the Aquaporin family Water crosses cell membranes by two routes: diffusion through the lipid bilayer and through water channels called aquaporins The aquaporins are a family of membrane channel proteins that serve as selective pores through which water crosses the plasma membranes of many human tissues and cell types. 6) Compare and contrast preimplantaion embryonic cells and cancer cells Human pre implantation embryonic cells are similar in phenotype to cancer cells. Both types of cell undergo deprogramming to a proliferative stem cell state and become potentially immortal and invasive. Both embryonic stem cells and cancer cells are (1) rapid-growing and (2) usually undifferentiated. Undifferentiated means that they are very primative cells which have not acquired a specific function such as nerve cells, muscle cells, lung cells, etc. 1) Both of them go through Epithelial-mesenchymal transitions (EMT): Embryonic cells: In order for the cells to move from the epithelium of the epiblast through the primitive streak to form a new layer, the cells must undergo an epithelial to mesenchymal transition (EMT) to lose their epithelial characteristics, such as cell-cell adhesion. Along the primitive streak, a small number of cells in the tight epithelial structures, the cell adhesion junctions, are released and the cells move closer together inwards forming mesenchymal cells. Cancer cells: EMT also important in cancer in which some of the cells lose their tight cell-cell interactions and migrate away from the tumor, go into the blood and metastasise to other organs. 2-The Warburg effect: embryonic and cancer cells both express this whereas normal or adult cells don’t. Embryonic cells express pyruvate kinase M2 and are very dependent upon their expression. Tumor cells are also dependant upon expression of pyruvate kinase M2, tumor cells are highly metabolically active and thus go through glycolysis. Both embryonic cells and cancer cells die if PKM2 was inhibited. 3-Defect in restricting cell lineage specification, master gene expression, cell proliferation can all lead to cancer. 4-Many “developmental genes” are expressed again during cancer: Many of the genes that are on during embryonic development are switched on again during tumorogenisis such as SCL (stem cell leukemia) normally expressed in embryo shouldn’t be expressed in adults). 7) Following a mutation in KCNE1 in which it is no longer expressed, explain the physiological effects Potassium voltage-gated channel - mutations in the KCNE1 gene Mutations on this gene lead to a loss of function and a loss of voltage dependence. These mutations change a single amino acid in the KCNE1 protein, which disrupts the protein's normal structure. An altered KCNE1 protein cannot regulate the flow of potassium ions through channels in cardiac muscle. This loss of channel function leads to abnormal heart rhythm (arrhythmia). This gene is also found in the ear, but a mutation in it has no affect on hearing. - premature stop codon Here, there’s no channel expressed leading to long QT syndrome increasing the risk of cardiac arrest and sudden death. People with this condition will be deaf! 8) a) Define the term Hayflickness of cells “Hayflickness” refers to the finite replicative potential (number of cell generations) (100) that limits total cell lifespan in most normal somatic cells, important in preventing the accumulation of mutations and protecting against oncogenesis. SO it is the number of times a normal cell population will divide before it stops, presumably because the telomeres reach a critical length b) Explain what a telomere is and how it can be extended and formed by telomerase Telomeres are tandem repeats of non-coding DNA at chromosome ends. Telomeres shorten at each round of replication (due to incomplete lagging strand synthesis - no place from which to produce RNA primer when reach chromosome end). Telomeres bind capping proteins that fold chromosome ends into t-loop, protecting single strand overlap from degradation. Telomeres are tandom repeats of non-coding DNA at chromosome ends. Telomeres shorten after each round of replication. After each replication, a portion of telomere is lost due to incomplete lagging strand synthesis. As a result, there would be no place from which to produce RNA when reach chromosome ends. Telomeres act to protect chromosome ends; they do so by binding capping proteins (shelterin complex) that protect chromosome ends and prevent degradation. When telomeres become very short, cells go to senescence. However, in the presence of the enzyme telomerase, cells continue replication and do not enter senescence. Telomerase is an enzyme that has a short piece of RNA embedded in it and is capable of stabilising telomere length by adding DNA repeats (TTAGGG) to the DNA strands. Telomere is thus extended by RNA template DNA synthesis. This region of repeated nucleotide called telomeres contains non-coding DNA material and prevents constant loss of important DNA from chromosome ends. As a result, every time the chromosome is copied, telomere length is maintained which causes no damage to the organism's DNA. c) Explain why telomerase expression may allow tumourogenesis As mentioned above, telomere length is stabilised by telomerase which adds DNA repeats to the chromosome ends. As a result of telomere maintenance, cells no longer go to senescence but instead they replicate indefinitely to achieve immortality. That is chromosomes will not become unstable no matter how many cell divisions they undergo. However, telomerase expression is not a cancer forming event alone; it requires an oncogenic change. In order for the cell to go to immortality, both checkpoint failure and telomerase activation are required. After checkpoint failure, if telomerase is not present, the cells go to crisis and then to apoptosis, whereas if the telomerase was active, the telomere and chromosome are stabilised and result in cell survival and immortality. Thus the cooperation of both telomerase activation and checkpoint failure are required for tumorogenesis. The majority of human tumor cells acquire immortality through expression of the catalytic subunit of telomerase (hTERT). Once immortalized, human cells are susceptible to transformation by introduction of an oncogene such as ras 9) Draw a diagram outlining the cell cycle with regards to the specific cyclin complexes with each phase and the cyclins involved in progression past any restriction points 10) 11a) Define the definitive ectoderm, neurectoderm and embryonic stem cells in terms of their proliferation and differentiation capacities The definitive ectoderm gives rise to the surface ectoderm and neural ectoderm. It is multipotent Neuroectoderm: the portion of the ectoderm of the early embryo that gives rise to the central and peripheral nervous systems, includes neurons and glia Embryonic stem cells (ES cells) are stem cells derived from the inner cell mass of the blastocyst. ES cells are pluripotent, that is, they are able to differentiate into all derivatives of the three primary germ layers: ectoderm, endoderm, and mesoderm. These can give rise to any cell in the body Primitive ectoderm: primitive ectoderm is formed from the inner cell mass. It requires 4 factors: 1) Autocrine signalling from the cells of the inner cell mass (required for pluripotency). 2) Paracrine signalling- signalling from one cell to another. (Visceral endoderm to ICM; which is critical for embryonic development). (required for differentiation) 3) Cell-basement membrane adhesion. Cells in the ICM express integrins and these can bind to the proteins of the ECM (required for survival and proliferation) 4) Cell-cell contact adhesion You can go into alot more details from Michael morris’s lectures. The cells that not in direct contact with the ECM, only receive pluripotent signals because they are away from the ECM and VE and as a result die and form a cavity whereas cells that are close to the ECM receive all the signals and survive and therefore turn into primitive ectoderm. Primitive ectoderm is important because it’s the substrate for gastrulation and forms the multipotent germ layers; ectoderm, endoderm, and mesoderm. Neuroectoderm Neurulation occurs when ectoderm starts to invaginate to form the neural plate and then the neural groove and this neural groove keeps on invaginating deeper to form the neuroectoderm in the inside, however on the outside the epidermal ectoderm is formed. Neuroectoderm then goes and gives rise to the neural tube (CNS, brain, retina..) and neural crest cells. We can go into much more details (Michael morris first lecture) b) Explain their differences in terms of the type of markers they express and their properties How to identify different cell types: - Can inject es cells into a blastocyst to form a chimera which is a measure or pluripotency. The injected cells end up in every tissue of the adult. Es cells do form a chimera Take es cells and inject them under a kidney capsule of a mouse, and you get teratomas (contains cells derived from all germ layers). Property of pluripotent cells. Another way is to look at marker expressions that are expressed specifically in a particular cell type. As shown in the table below, only es cells express markers of pluripotency ie Oct4 and Alkaline phosphatase. The definitive ectoderm and the neuroectoderm are both multipotent and don’t express those markers. Some markers are es cells specific such as Nanog. If you want to look at multipotent cell types like definitive ectoderm and neuroectoderm you can use other markers. Eg: Sox1 is neuroectoderm specific. ESC Express markers of pluripotency? Oct4 Y Alk. Phos Y Other markers Nanog Y Fgf5 N Sox1 N Sox2 Y NeuN N Definitive Ectoderm Neuroectoderm N N N No N N N Y N N N Y Y N The Western blot (alternatively, protein immunoblot) is a widely used analytical technique used to detect specific proteins in the given sample of tissue homogenate or extract. It uses gel electrophoresis to separate native or denatured proteins by the length of the polypeptide (denaturing conditions) or by the 3-D structure of the protein (native/ non-denaturing conditions). The proteins are then transferred to a membrane (typically nitrocellulose or PVDF), where they are probed (detected) using antibodies specific to the target protein. Immunoprecipitation is a method that enables the purification of a protein. An antibody for the protein of interest is incubated with a cell extract so that the antibody will bind the protein in solution. The antibody/antigen complex will then be pulled out of the sample using protein A/G-coupled agarose beads. This physically isolates the protein of interest from the rest of the sample. The sample can then be separated by SDS-PAGE for Western blot analysis. Creation of proteins The proteins are created in the cells by the ribosome by a process called “Translation” in which a mRNA molecule (that was transcript from the DNA inside the nucleus) comes out of the nucleus to the Cytoplasm, this mRNA molecule carry the genetic code of the DNA and proteins are translated in the cytoplasm. In this process is involved another protein molecule called Ribosome. Recombinant protein Types Recombinant protein is a protein that it’s code was carried by a recombinant DNA. The term recombinant DNA means that two segments of DNA in a plasmid. Plasmids are usually occurring in bacteria. When a recombinant DNA is inserted to a bacteria, this bacteria will produce protein based on this recombinant DNA. This protein is called recombinant protein. The use of bacteria in order to produce recombinant protein has grown in the recent years. Using recombinant DNA and inserting it to a plasmid of rapidly reproducing bacteria enables the manufacture of recombinant protein. These recombinant proteins can be variety of types, the can be Antibodies, antigens, hormones and enzymes.