Why are valence electrons important?
advertisement

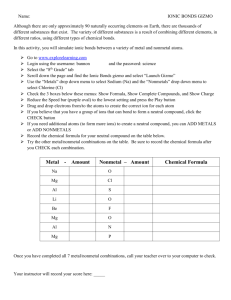
I. Introduction A. What is a bond? A mutual attraction between the electrons of 2 atoms that binds them together B. How do bonds form? Electrons are either shared or transferred between atoms C. Terms: a. Energy Levels: The “shells” of an atom where the electrons are located (found from the rows on the periodic table) b. Valence electrons: the outer shell electrons (ones on the outer most energy level) D. Atom Pictures 1. Hydrogen 2. Helium D. Atom Pictures 3. Lithium 4. Beryllium **Why are valence electrons important? II. Lewis Structures A. What is a Lewis Structure? A way to represent the valence electrons in an atom B. Basic Notation: II. Lewis Structures C. How to find the number of valence electrons for main group elements: Look at the columns (groups) on the periodic table. Only works for main group elements Column # Valence Electrons 1 1 2 2 13 Transition Metals (vary – will be given) 3 14 4 15 5 16 6 17 7 18 8 (He 2) Element # of valence electrons Lewis Dot Structure Element Hydrogen Aluminum Helium Chlorine Sodium Oxygen Carbon Krypton # of valence electrons Lewis Dot Structure Warm-up 1/31/13 Element 1. Sulfur 2. Calcium 3. Nitrogen 4. Lithium 5. Argon 6. Rubidium # of Valence electrons Lewis structure Warm-up 2/1/13 Element 1. Sulfur 2. Nitrogen 3. Rubidium 4. Argon # of Valence electrons Lewis structure Why do elements form compounds? To become more stable elements Octet Rule: All atoms want a full outer shell. Most want 8 (oct = 8), H wants 2 **All elements want a ____________________ and be like a _______________________. III. Ionic Bonds: Transfer of electrons between a metal and a nonmetal A. Terms: a. Ion: An element with an overall charge (unequal numbers for p+ and e-) b. Cation: A positive ion c. Anion: A negative ion A.Lewis structure to ion: Element Lithium Sodium Magnesiu m Calcium Oxygen Sulfur Fluorine Chlorine Gain/Lose? Lewis structure How Many? Charge Metal/ Nonmetal ? Cation/ Anion? B. Properties of ionic bonds: • • • • Form solid crystals at room temperature Hard, brittle High melting temperatures (boiling points) Tend to dissolve in water (breaks into ions) – Conducts electricity when melted or dissolved in water (electrolyte). Solids do not conduct. Properties How Salt Dissolves in Water C. Forming Ionic Bonds 1) GOAL: All atoms have a full outer shell 2) Metals lose electrons to become like noble gases. 3) Nonmetals gain electrons to become like noble gases 4) Each element wants a full outer shell Transfer of Electrons 5) Lewis Structure Examples: Elements Lithium and Bromine Calcium and Oxygen Sodium and Oxygen Metal Lewis Structure Nonmetal Lewis Structure Ionic Bond 6) Charge examples: Elements Lithium and Bromine Calcium and Oxygen Sodium and Oxygen Metal Charge Nonmetal Charge Ionic Bond Ionic Exit 1/31/13: 1. Show how potassium and iodine bonds. 2. Show how rubidium and oxygen bonds. 3. Show how aluminum and fluorine bonds. Warm-up 2/4/13 1. Show how lithium and fluorine bond 2. Show how aluminum and oxygen bond D. Naming Ionic Compounds a. RULES FOR NAME TO FORMULA 1. Write the metal and charge first (if RN – that is the charge on the metal) 2. Write the nonmetal and charge second 3. Cross and bring down numbers; remove charges and CHECK! HINT: All charges should add to zero b. EXAMPLES 1.Sodium chloride 2. Iron (III) oxide 3. Potassium oxide 4. Tin (IV) selenide 5. Aluminum sulfide Practice 1 1. 2. 3. 4. 5. Aluminum fluoride Calcium bromide Iron (III) chloride Silver (I) chloride Lead (II) oxide c. RULES FOR FORMULA TO NAME 1. Name the metal (cation) first a. If a transition metal, write roman numeral (need to figure out charge) 2. Name the nonmetal (anion) second a. Change ending to -ide d. EXAMPLES 1.NaF 2. MgO 3. CaCl2 4. CuBr2 5. Al2O3 Practice 2 1. 2. 3. 4. 5. MgBr2 Cs2O TiF3 NiO AuCl3 E. Naming with Polyatomic Ions a. Polyatomic Ion: • Covalently bonded molecules with a charge • Combine with metals to form ionic compounds b. Follow rules for naming ionic compounds; be aware that compounds with polyatomic ions will have more than 2 elements. Pictures c. Examples: Name to Formula: 1.Sodium hydroxide 2. Calcium phosphate 3. sodium carbonate 4. Aluminum nitrate 5. Calcium hydroxide Formula to Name: 1.KMnO4 2. NaCH3COO 3. Al2(SO4)3 4. Ca(CN)2 5. NaOH Practice 3 1. 2. 3. 4. 5. Gold (III) cyanide Silver (I) nitrate Tin (IV) sulfate Sodium acetate Ammonium hydroxide 1. 2. 3. 4. 5. KClO4 Li2Cr2O7 (NH4)2S NaHCO3 Na2CO3 IV. Covalent Bonds: Sharing of electrons between 2 nonmetals. A.Terms: a. Polar: Unequal sharing of electrons (think “pulling”) b. Nonpolar: Equal sharing of electrons (think “not pulling”) c. Review: Remember – like dissolves like B. Properties of Covalent Bonds • May be solids, liquids or gases at room temperature • Most have low melting points (boiling points) – There are exceptions (EX: diamonds) • Poor conductors of heat and electricity – do not conduct (well or not at all) when dissolved in water C. Forming Covalent Bonds 1) GOAL: Atoms will share electrons to get a full outer shell 2) Exceptions to Octet Rule: H only wants 2 eB only wants 6 eS and P can have more than 8 Molecule H2O (water) CF4 (carbon tetrafluoride) Total valence electrons Lewis Structure D. Naming Covalent Compounds GREEK PREFIXES (starter) Number Prefix 1 Mono- 2 Di- 3 Tri- 4 Tetra- 5 Penta- 6 Hexa- 7 Hepta- 8 Octa- 9 Nona- 10 Deca- Other uses for these prefixes a. RULES FOR NAME TO FORMULA 1. 2. 3. 4. Write the symbol for the first element Look at the prefix – this is the subscript Write the symbol for the second element Look at the prefix – this is the subscript b. EXAMPLES 1.Nitrogen tribromide 2. Dinitrogen trisulfide 3. Carbon monoxide 4. Tetraphosphorus decoxide 5. Carbon tetrahydride 6. Diboron hexahydride c. RULES FOR FORMULA TO NAME 1. Name the first element a. Use the subscript to determine the Greek Prefix b. No prefix needed for only one atom 2. Name second element a. Use subscript to determine the prefix (always) b. Change ending to -ide d. EXAMPLES 1.CO2 2. H2O 3. P2O5 4. CCl4 5. CF4 REVIEW: Describe each type of bond shown in the picture below: V. Metallic Bonds: a lattice of positive ions in a sea of electrons A. Properties: • Good Conductors of heat and electricity • Malleable (flattened into sheets) • Ductile (pulled into wires) • Solids at room temperature (except Mercury) Video IV. Examples a. Classify the following compounds as having Ionic (I), Covalent (C), or Metallic (M) bond. a.CuCl2 b.CO2 c.Ag d.NaCl e.O2 f.Fe2O3 g.H2O h. Na i.CaF2 j.H2 ________ ________ ________ ________ ________ ________ ________ ________ ________ ________ Review – Exit Type of bond Between metals and/or nonmetals Ionic Covalent Metallic 1. What is a cation? 2. What is a anion? Electrons…