3x10 8
advertisement

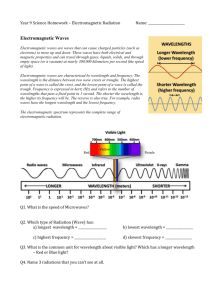
Pop Quiz On a piece of paper write your name and the answers to the following questions. When you are finished place your answers in the tray on my desk. You have 10 minutes •What are the prefixes represented by the ‘King Henry Died by drinking chocolate milk’ acronym. 7 points •Convert the following into meters. Use scientific notation 475nm 4.75x10-7m 25cm 1500km =2.5x10-1m 1.5x106m 3 points Electromagnetic Spectrum Wavelength ‘The distance between successive crests of a wave’ Frequency ‘the number of periodic oscillations, vibrations or waves occurring per unit of time’ Relationships frequency, (s-1) velocity, (m/s) c=λν wavelength, (m) Wave Particle Duality Electromagnetic radiation fits the rules of behavior for both waves and particles. This is known as waveparticle duality The reason for this is that just thinking of light (or any type of radiation) as a wave doesn’t explain certain things. Task: Use p84 of the black study guide to make notes of wave- particle duality. You have 10 minutes The energy of a photon (a particle of light or electromagnetic radiation) can be calculated using the following formula Planck’s constant, 6.63x10-34 Js E=hν Energy, kJ/mol Frequency, s-1 As you can see energy is directly related to frequency Questions: Which of the following has a longer wavelength? •Red light or blue light? •Microwaves or radio waves? •Infrared radiation or red light? •Gamma rays or uv radiation? Which of the following has a greater frequency? •Yellow light or green light? •X-rays or gamma rays? •UV radiation or violet light •AM radio waves or FM radio waves Which of the following has the lower energy? (remember energy is directly related to frequency) •Red light or blue light? •Microwaves or radio waves? •Gamma rays or uv radiation? •UV radiation or violet light? Springfields ‘Classic Rock’ radio station broadcasts at a frequency of 102.1 MHz. What is the length of the radio wave in meters? (1 MHz = 1x106 Hz) c=λν 3x108 λ = 3x108 = 102.1x106 = 2.94m λ x 102.1x106 A beam of light has a wavelength of 506 nanometres. What is the frequency of the light? (1nm= 1x10-9m) What colour is the light? c=λν 3x108 ν = 506x10-9 3x108 = 506x10-9 = 5.93 x1014Hz x ν Blue light has a frequency of 6.98x1014 Hz. Calculate the wavelength of blue light c=λν 3x108 3x108 λ = 6.98x1014 = 4.30x10-7m or 430nm 14 x 6.98x10 = λ Now complete the worksheet. It should be handed in at the end of the lesson Homework: Answer q5&6 on p126 0f the textbook