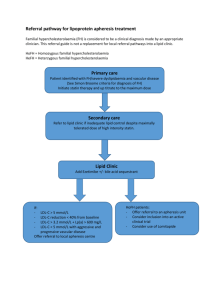
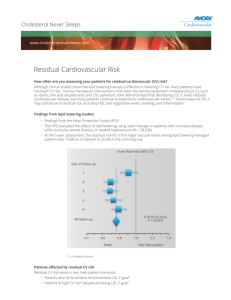
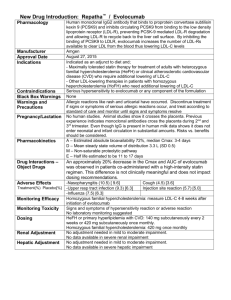
William Cromwell, MD: Risk factors in heart disease – Optimizing
advertisement

Risk factors in heart disease Optimizing patient care William Cromwell, MD, FAHA, FNLA Chief Medical Officer – LipoScience, Inc. Chief – Lipoprotein and Metabolic Disorders Institute Adjunct Associate Professor – Wake Forest University School of Medicine Disclosures William Cromwell, MD, FAHA, FNLA Current Perspectives on LDL Management 1. The causal link between high levels of low-density lipoprotein (LDL) and the development of CVD is well established 1 Increased numbers of circulating LDL particles accelerates development of atherosclerotic cardiovascular disease The longer the exposure to high LDL, the greater the risk of CVD events 2. Lowering LDL is a central tenet of clinical practice 2013 ACC/AHA guidelines recommend a two step approach to managing LDL-related CVD risk 1 - Use moderate or high dose statin therapy in selected populations; - Monitor LDL levels on therapy and use clinical judgment in determining next steps in patient management. 1. Stone NJ, et al. Circulation 2014;129:S1-S45. 2. Otvos JD, et al. Am J Cardiol. 2002;90(8A):22i-29i. 3. Cromwell WC, Otvos JD. Am J Cardiol. 2006;98(12):1599-1602. 4. Cromwell WC, et al.. J Clin Lipidol. 2007;1(6):583-592. 5. Otvos JD, et al. J Clin Lipidol. 2011;5(2):105-113. 6. Sniderman AD, et al. Am J Cardiol. 2003;91(10):1173-1177. 7. Sniderman AD, et al. Am J Cardiol. 2001;87(6):792-793, A798. 8. Sniderman AD. J Clin Lipidol. 2008;2(1):36-42. Two Ways To Measure LDL Quantity LDL cholesterol (LDL-C) is the traditional measure of LDL, chosen for historical, not analytic or clinical reasons. Alternatively, LDL can be measured by particle number (LDL-P), or estimated by apolipoprotein B. Due to differences in the amount of cholesterol contained in LDL, alternate LDL measures (LDL-C vs. LDL-P) frequently disagree (discordance).1-7 Triglycerides Triglycerides LDL Particle LDL Particle LDL-P LDL-P LDL-C LDL-C Cholesterol Cholesterol 1. Otvos JD, et al. Am J Cardiol. 2002;90(8A):22i-29i. 2. Cromwell WC, Otvos JD. Am J Cardiol. 2006;98(12):1599-1602. 3. Cromwell WC, et al.. J Clin Lipidol. 2007;1(6):583-592. 4. Otvos JD, et al. J Clin Lipidol. 2011;5(2):105-113. 5. Sniderman AD, et al. Am J Cardiol. 2003;91(10):1173-1177. 6. Sniderman AD, et al. Am J Cardiol. 2001;87(6):792-793, A798. 7. Sniderman AD. J Clin Lipidol. 2008;2(1):36-42. Alternate LDL Measures (LDL-C versus LDL-P) Multi Ethnic Study of Atherosclerosis [MESA] (n=6,697) Otvos et al. J Clin Lipidol 2011;5:105-13 Alternate LDL Measures (LDL-C versus LDL-P) Multi Ethnic Study of Atherosclerosis [MESA] (n=6,697) Discordant Measures LDL-C (mg/dL) 79 LDL-C and LDL-P Different (50% Subjects) 90 100 108 116 123 131 141 157 LDL-P > LDL-C LDL-P percentile 80 Concordant Measures 1750 Less Cholesterol per Particle 1580 70 1460 60 1360 50 1270 40 1190 30 1100 20 LDL-P < LDL-C 1000 10 More Cholesterol per Particle 880 LDL-C and LDL-P Similar (50% Subjects) Otvos et al. J Clin Lipidol 2011;5:105-13 10 20 30 40 50 60 70 LDL-C percentile 80 90 LDL-P (nmol/L) 90 Alternate LDL Measures (LDL-C versus LDL-P) Type II Diabetes Mellitus Subjects (n=2,355) 5th 20 1% (n=19) 20th 24% (n=364) 50th 43% (n=631) 80th 21% (n=307) percentile 11% (n=163) 15 Percent of Subjects LDL-C 70-99 mg/dL (5th – 20th Percentile) 10 5 (n=1,484) 0 700 20 16% (n=147) 1000 43% (n=377) 1300 30% (n=260) 1600 (nmol/L) 9% (n=76) 2% (n=15) 15 Percent of Subjects 40% LDL-C 10 < 70 mg/dL (< 5th Percentile) 5 (n=871) 0 700 Cromwell WC, Otvos JD. AJC 2006;98:1599-1602 1000 1300 1600 (nmol/L) Alternate LDL Measures and Cardiovascular Disease 1. Cardiovascular risk tracks with LDL particle number – When alternate LDL measures (LDL-C vs LDL particle number) agree (concordance) each measure is equally associated with CVD risk. – When alternate measures are discordant (e.g., diabetes, metabolic syndrome, statin therapy), risk tracks with LDL-P, not LDL-C.1-5 1. Cromwell WC, et al.. J Clin Lipidol. 2007;1(6):583-592. 2. Otvos JD, et al. J Clin Lipidol. 2011;5(2):105-113. 3. Sniderman AD, et al. Am J Cardiol. 2003;91(10):1173-1177. 4. Sniderman AD, et al. Circ Cardio quality and outcomes. 2011;4(3):337-345. 5. Sniderman AD, et al. Atherosclerosis. Dec 2012;225(2):444-449. Associations of Alternate LDL Measures with CHD Framingham Offspring Study (n=3,066) 1.00 Better survival Lower risk 0.98 0.96 Low LDL-C Low LDL-P (n=1,249) 0.94 Event-Free Survival 0.92 Worse survival Higher risk 0.90 0.88 High LDL-C Low LDL-P (n=284) High LDL-C High LDL-P (n=1,251) 0.86 0.84 Better Survival Lower Risk Worse Survival Higher Risk 0.82 Low LDL-C High LDL-P (n=282) 0.80 0.78 Concordant 0.76 Discordant 0.74 0 1 2 3 4 5 6 7 8 9 Years of Follow-up Cromwell WC et al. J Clin Lipidol 2007;1(6):583-592. 10 11 12 13 14 15 16 LDL-P and LDL-C Discordance in MESA Relations with Incident CVD Events 0.08 Percent Cumulative Incidence Cumulative Incidence MetSyn LDL-P >> LDL-C LDL-C LDL-P Concordant Concordant 0.06 60.06 LDL-P << LDL-C LDL-C LDL-P 54% 33% 16% LDL-C underestimates LDL-attributable risk 0.04 40.04 0.02 20.02 3 4 22 2 3 4 3 Follow-up (years) Follow-up (years) Otvos et al. J Clin Lipidol 2011;5:105-13 4 5 5 5 104 1372 117 1249 1117 mg/dL nmol/L 0 111 LDL-P 130 LDL-C overestimates LDL-attributable risk 0 00 LDL-C 6 High LDL Despite Low LDL-C Low LDL Despite High LDL-C LDL-P and LDL-C Discordance in MESA CVD Event Rates in Subgroups with Low LDL-C Incidence Cumulative Incidence Percent Cumulative 0.08 Low: < 30th percentile LDL-C < 100 mg/dL LDL-P < 1060 nmol/L 0.06 60.06 LDL-P LDL-C (n) Not Low Low (516) Discordant High LDL-P 0.04 40.04 Concordant Low 0.02 20.02 00 0 0 Otvos et al. J Clin Lipidol 2011;5:105-13 1 1 2 3 4 2 3 4 Follow-up (years) 5 5 6 Low (1115) LDL-P and LDL-C Discordance in MESA CVD Event Rates in Subgroups with Low LDL-P 1 Incidence Cumulative Incidence Percent Cumulative 0.08 Low: < 30th percentile LDL-C < 100 mg/dL LDL-P < 1060 nmol/L 0.06 60.06 LDL-P LDL-C (n) Not Low Low (516) Discordant High LDL-P ACC/AHA Threshold for Considering Statin Therapy (7.5% risk over 10 years) 2 0.04 4 0.04 Concordant 0.02 20.02 Low Low Low Not Low (553) 00 0 0 1 1 1. Otvos et al. J Clin Lipidol 2011;5:105-13 2. Adapted from Stone et al. Circulation. 2013 2 3 4 4 3 2 Follow-up (years) 5 5 6 (1115) A Meta-Analysis of Low-Density Lipoprotein Cholesterol, Non-High-Density Lipoprotein Cholesterol, and Apolipoprotein B as Markers of Cardiovascular Risk Allan D. Sniderman, MD; Ken Williams, MSc; John H. Contois, PhD; Howard M. Monroe, PhD; Matthew J. McQueen, MBChB, PhD; Jacqueline de Graaf, MD, PhD; Curt D. Furberg, MD, PhD Circulation. Cardiovascular quality and outcomes. 2011;4(3):337-345. Meta-Analysis of LDL-C, Non-HDL-C, and ApoB as Markers of Cardiovascular Risk Study Design: Meta-analysis of all published epidemiologic studies with estimates of relative risks of fatal or nonfatal ischemic cardiovascular events and measures of non-HDL-C and apoB. 12 independent reports, including 233,455 subjects and 22,950 events, were analyzed. Major Findings: Whether analyzed individually or in head-to-head comparisons, apoB was the most potent marker of cardiovascular risk. Sniderman AD, Williams K, et al. Circulation. Cardiovascular quality and outcomes. 2011;4(3):337-345. Meta-Analysis of LDL-C, Non-HDL-C, and ApoB as Markers of Cardiovascular Risk Conclusions: “The present analysis indicates that non-HDL-C is superior to LDL-C as a marker of cardiovascular risk.” “The conventional explanation would be that the gain in predictive power is due to the cholesterol in VLDL.” “The superiority of non-HDL-C over LDL-C is due to the fact that nonHDL-C is a better marker of LDL-P than LDL-C.” “When apoB and non-HDL-C are concordant, they will predict risk equally, whereas when they are discordant, apoB will be superior.” Sniderman AD, Williams K, et al. Circulation. Cardiovascular quality and outcomes. 2011;4(3):337-345. LDL Subclasses: 2011 National Lipid Association Recommendations “Many studies document links between small dense LDL particles and atherosclerotic CVD.” “However, these statistical associations between small, dense LDL and CV outcomes are either significantly attenuated or abolished when the analyses are adjusted for the overall number of circulating LDL particles (LDL-P) either by adjustment for Apo B levels or by adjustment for nuclear magnetic resonance-derived LDL-P.” Adapted from Davidson, et al. J Clin Lipidol 2011;5:338-367. LDL Subclasses: 2011 National Lipid Association Recommendations “To date, there is no evidence that the shift in LDL subfractions directly translates into change in disease progression or improved outcome.” “The NLA Biomarkers Expert Panel was unable to identify any patient subgroups in which LDL subfractionation is recommended.” Adapted from Davidson, et al. J Clin Lipidol 2011;5:338-367. Expectations for Novel Risk Tests versus An Alternate Measure of an Established Target Intended Application Type of Biomarker Clinical Use Impact on Clinical Decision Making Evidence Needed to Support Use Biomarker is used to enhance risk assessment Allocate patient to different risk category Significant improvement in risk stratification with the addition of new biomarker (net reclassification) 1 Novel Biomarker Risk Assessment (lipoprotein particle size / subclasses, Inflammatory measures) Novel Biomarker (LDL particle size, Inflammatory measures) Risk Management Newer Measure of an Established Target Biomarker serves as a treatment goal (e.g., LDL particle number) 1, Ge Y, Wang TJ. J Intern Med. 2012;272(5):430-439. 2. Glasziou P, et al. Ann Intern Med. 2008;149(11):816-822 Modify therapy (different agents, dosage, or combinations) as indicated to achieve new therapeutic goal Outcome improvement established independent of other risk factors 1. Newer measure consistently outperforms the old measure in the setting of discordance.2 2. Improved outcomes are noted when subjects are treated to equivalent levels of the new versus old measure. Recommendations for Using LDL Particle Number Measures as Targets of Therapy Recommendations for Using LDL Particle Number Measures as Targets of Therapy Step 1: Stratify ASCVD risk and initiate therapy (Statin therapy if triglyceride levels < 500 mg/dL) Step 2: Assess adequacy (laboratory testing) and tolerance of therapy Risk Level Moderate Risk High Risk DM but no other major risk and/or age < 40 DM + major CVD risk(s) (HTN, Family History, Low HDL-C, smoking) or CVD Desirable Level LDL-P (nmol/L) < 1200 < 1000 apoB (mg/dL) < 90 < 80 Step 3: If not at desired level intensify therapeutic lifestyle, consider additional therapy Intensify statin therapy; Consider combination statin &/or ezetimibe &/or colesevelam &/or niacin Step 4: Assess adequacy / tolerance of therapy with and consider additional therapeutic adjustment. Adapted from Garber AJ, et al. Endocr Pract 2013;19 (Suppl 2):1-48. Recommendations for Using LDL Particle Number Measures as Targets of Therapy • “These data indicate that both Apo B and LDL-P were generally in agreement in their association with diverse clinical outcomes (58.8%), but with a substantial amount of discordance (21.2%) in which one biomarker was statistically significant whereas the other was not.” • “In these cases, LDL-P showed a significant association with a clinical outcome more often than apo B alone, and the level of statistical significance, as indicated by the P value, and the strength of association, as indicated by the OR, RR, and HR, was more often higher for LDL-P than it was for apo B.” Cole TG, et al. Clinical Chemistry February 2013;59(5):752-770 2013 ACC / AHA Cholesterol Guidelines 2013 ACC / AHA Cholesterol Guidelines Overview 1. Objective: Produce treatment recommendations, based on randomized controlled trial (RCT) data, to reduce atherosclerotic cardiovascular disease (ASCVD) risk. 2. Based on RCT data significant emphasis was placed on identifying populations most likely to benefit from statin therapy. “Because the overwhelming body of evidence came from statin RCTs, the Expert Panel appropriately focused on these statin RCTs to develop evidence-based guidelines for the reduction of ASCVD risk.” 1 1. Stone et al. Circulation. 2013 ASCVD Statin Benefit Groups No Stone et al. Circulation. 2013 2013 ACC / AHA Cholesterol Guidelines Role of LDL Testing 1. While acknowledging the causal role of LDL in ASCVD, due to exclusive reliance on RCT data no recommendation was made for LDL treatment goals. “The panel makes no recommendations for or against specific LDL-C or nonHDL-C targets for the primary or secondary prevention of ASCVD.” 1 2. Although no goal is endorsed, LDL testing is advocated to aid clinical management – ATP III Recommendation: LDL testing was used to achieve risk-based LDL goal – 2013 ACC/AHA Recommendation: LDL testing is used to monitor therapeutic response and adherence 3. Modifying individual treatment requires clinical judgment. “The ultimate decision about care of a particular patient must be made by the healthcare provider and patient in light of the circumstances presented by that patient.” 1 1. Stone et al. Circulation. 2013 Statin Therapy: Monitoring Therapeutic Response and Adherence Stone et al. Circulation. 2013 Integrating Population Based and Individual Optimization Strategies in Practice 1. The 2013 ACC/AHA Guideline is a starting point for population management, but is not an end point for individual care. This highlights two different opportunities to improve patient care: - Population strategy (A) – treat population with generalized therapy to achieve relative risk reduction among the group - Individual optimization strategy (B) – monitor individual response with a reliable LDL measure and adjust care as indicated. 2013 Guidelines advise clinicians to integrate these options: - Use of A and B (start with population care, followed by individual optimization based on clinical judgment) is recommended; - Use of A only (population strategy, “Fire and Forget”) is not advised. 2. Exclusive use of a population strategy is incapable of judging individual response to statin therapy or optimizing individual management. Heterogeneous Response to High Intensity Statin Therapy Cardiovascular Events In Treat to New Target “TNT” Patients with major CVD events (%) 18 Atorva 10 mg 16 Atorvastatin 10 mg (LDL-C on-trial 101 mg/dL) Atorva 80 mg 14 Atorvastatin 80 mg 12 (LDL-C on-trial 77 mg/dL) No Benefit From Aggressive Treatment (44 %) 10 22% Reduction in Major cardiovascular events (p=0.0002) 8 6 2 56% Benefited From High Intensity Statin Therapy 0 WHY? 4 0 1 2 3 # MetSyn Components Deedwania P, et al. The Lancet. 2006;368:919-928 4 5 Potential Answer to TNT is Supplied by Framingham 180 N=286 N=407 N=355 N=233 N=113 N=30 1800 LDL-C 170 1700 160 1600 150 1500 140 1400 130 1300 120 1200 110 1100 0 1 2 MetSyn (-) Kathiresan S, et al. Circulation 2006;113:20-27 3 4 MetSyn (+) 2.3x risk 5 LDL-P (nmol/L) LDL-C (mg/dL) LDL-P With Higher LDL-P, Greater Benefit Is Expected From More Intensive LDL-P Lowering. Relations of Change in Plasma Levels of LDL-C, Non-HDL-C and apoB With Risk Reduction From Statin Therapy: A MetaAnalysis of Randomized Trials George Thanassoulis, Ken Williams, Keying Ye, Robert Brook, Patrick Couture, Patrick R. Lawler, Jecqueline de Graaf, Curt D. Furgerg and Allan Sniderman Journal of American Heart Association 2014;3 Meta-Analysis of LDL Measures and Risk Reduction from Statin Therapy Objective: • To evaluate the relationship between the reduction in alternate LDL measures (LDL-C, non-HDL-C, apoB) and observed cardiovascular benefit produced by statin therapy in randomized, placebo controlled trials. – “The marker whose reduction relates most directly to benefit should also be the marker that is best to identify those whose outcome might be improved by further lipid lowering.” • Meta-analysis was performed using both frequentist and Bayesian methods. Thanassoulis G, et al. J Am Heart Assoc. 2014;3:e000759 Meta-Analysis of LDL Measures and Risk Reduction from Statin Therapy Studies Selected: Analyzed all published, placebo-controlled studies, which have reported baseline and on-treatment levels of LDL- C, non-HDL-C, and apoB. Thanassoulis G, et al. J Am Heart Assoc. 2014;3:e000759 Meta-Analysis of LDL Measures and Risk Reduction from Statin Therapy Findings: Relative risk reduction from statin therapy in the 7 major placebocontrolled statin trials demonstrated: – Risk reduction was more closely related to reductions in apoB than to reductions in either non-HDL-C or LDL-C. – Changes in non-HDL-C and LDL-C appeared to be statistically indistinguishable with respect to risk reduction of statin therapy. Within trial “head-to-head” comparisons of cardiovascular risk relationship with individual LDL markers : – LDL-C was 2.4% (- 3.6%, 8.4%) > non-HDL-C (P=0.445) – apoB was 21.6% (12.0%, 31.2%) > LDL-C (P<0.001) – apoB was 24.3% (22.4%, 26.2%) > non-HDL-C (P<0.001). Thanassoulis G, et al. J Am Heart Assoc. 2014;3:e000759 Cardiovascular Risk in Patients Achieving Low-Density Lipoprotein Cholesterol and Particle Targets Peter P. Toth , MD, PhD Michael Grabner , PhD Rajeshwari S. Punekar , PhD Ralph A. Quimbo , MA Mark J. Cziraky , PharmD Terry A. Jacobson , MD Atherosclerosis 2014;235(2):585-591 Study Design • Claims data between 2006 and 2012 were used to identify eligible patients achieving LDL-P <1000 nmol/L (LDL-P cohort) and patients achieving LDL-C<100 mg/dL (LDL-C cohort) without LDL-P measurements. • Demographic and comorbidity differences between the two cohorts were balanced using propensity score matching however, treatment patterns were left intact. Adapted from Toth PP, et al. Atherosclerosis 2014;235(2):585-591. Baseline Characteristics for Patients with ≥ 12 Months of Follow-Up Adapted from Toth PP, et al. Atherosclerosis 2014;235(2):585-591. Baseline Characteristics for Patients with ≥ 12 Months of Follow-Up Adapted from Toth PP, et al. Atherosclerosis 2014;235(2):585-591. Study Results At every follow-up interval the LDL-P cohort demonstrated: Significant risk reduction (Hazard Ratio): 24% at 12 months 22% at 24 months 25% at 36 months Significant event reduction (Number of patients with CHD/stroke events) Adapted from Toth PP, et al. Atherosclerosis 2014;235(2):585-591. 1.8% (8.12% - 6.26%) at 12 months 2.9% (13.9% - 11.0%) at 24 months 4.4% (19.0% - 14.6%) at 36 months Study Results Another metric of event reduction is the “Number Needed to Treat” (NNT). NNT = 1 / Event Reduction Represents the number of subjects needed to treat to prevent 1 event. (i.e., number of subjects needed to attain LDLP <1000 vs LDL-C <100 to prevent 1 CHD/stroke event). 2.9 Adapted from Toth PP, et al. Atherosclerosis 2014;235(2):585-591. Number Needed to Treat 2014;235(2):585-591. Approach to the Use of LDL in Clinical Practice Step 1: Stratify ASCVD risk (does not require LDL-P) Step 2: Institute appropriate course of treatment. Step 3: Use a reliable, FDA cleared, outcome proven LDL measure to monitor adherence and response among those treated. Step 4: Use clinical judgment in considering the need to modify individual therapy. Step 5: After modifying therapy, use a reliable, FDA cleared, outcome proven LDL measure to assess patient response. Use clinical judgment to consider modifications of treatment as indicated to optimize care. Selected Strategies to Reduce Particle Number Improve LDL Particle Clearance (remove more) Reduce LDL Particle Production (make less) Diet Exercise Weight Loss Glycemic Control Co-Morbidity Management (up to 30-50% 6 LDL-P) Marine Omega-3 o DHA + EPA (no 6 LDL-P) o EPA Only (4-15 % 6 LDL-P) LDL-P Target Statins (35-55% 6 LDL-P) Gut agents o Ezetimibe (15-30% 6 LDL-P) o Resins / Bile Acid Sequestrates (15-30% 6 LDL-P) Statin + Gut (50-70% 6 LDL-P) Statin + Gut + Niacin (> 60% 6 LDL-P) Adapted from Cromwell W, Dayspring T. Lipid and lipoprotein disorders: Current clinical solutions. Baltimore: International Guideline Center; 2012. Conclusions 1. Guidelines recommend a two step approach to managing LDL-related CVD risk: 1 Use moderate or high dose statin therapy in selected populations; Monitor LDL levels on therapy and use clinical judgment in determining next steps in patient management. 2. Because CVD risk tracks with apoB and NMR LDL-P 2-6, and because frequent discordance exists between LDL-C and measures of LDL-P 2-4,7-10, many expert panels advocate use of LDL particle number to adjudicate response and optimize individual therapy.11-13 3. Clinical utilization data confirms a significant reduction of CVD risk and events among high risk patients attaining low NMR LDL-P (mean 860 nmol/L) versus statin treated subjects with low LDL-C (mean 79 mg/dL).14 1. Stone NJ, et al. Circulation 2014;129:S1-S45. 2. Cromwell WC, et al.. J Clin Lipidol. 2007;1(6):583-592. 3. Otvos JD, et al. J Clin Lipidol. 2011;5(2):105-113. 4. Sniderman AD, et al. Am J Cardiol. 2003;91(10):1173-1177. 5. Sniderman AD, et al. Circ Cardio quality and outcomes. 2011;4(3):337-345. 6. Sniderman AD, et al. Atherosclerosis. Dec 2012;225(2):444-449. 7. Otvos JD, et al. Am J Cardiol. 2002;90(8A):22i-29i. 8. Sniderman AD, et al. Am J Cardiol. 2001;87(6):792-793, A798. 9. Cromwell WC, Otvos JD. Am J Cardiol. 2006;98(12):1599-1602. 10. Sniderman AD. J Clin Lipidol. 2008;2(1):36-42. 11. Contois JH et al. Clin Chem. 2009;55:407-419. 12. Davidson MH et al. J Clin Lipidol. 2011;5:338-367. 13. Garber AJ, et al. Endocr Pract 2013;19(Suppl 2):1-48. 14. Toth PP, et al. Atherosclerosis 2014;235(2):585-591.