Surface Plasmon Resonance - Electrical Engineering
advertisement
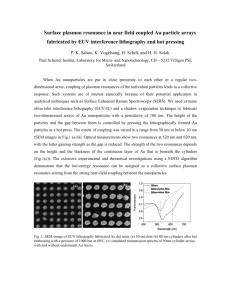
Surface Plasmon Resonance Portable Biochemical Sensing Systems Karl Booksh School of Biochemistry Arizona State University (Tempe) Denise Wilson Department of Electrical Engineering University of Washington (Seattle) National Science Foundation, Grant #ECS0300537 Surface Plasmon Resonance Portable Biochemical Sensing Systems ECS0300537 • The Big Picture – Why SPR? • • • • • Highly sensitive (10-4 to 10-6 RI units) Very local (10-100nm from sensing surface) Directly indicative (of interactions between sensor and environment) Relatively unencumbered by sampling overhead (e.g. tagging, mixing, etc) Readily referenced to compensate for background fluctuations (e.g. drift) – How is it used (SPR = transduction mechanism)? • Non-functionalized = bulk refractive index • Functionalized = specific analytes – The Full Spectrum of SPR-based instruments • User-Intensive, Single Measurements: Biacore • User-Intensive, Single Field Measurements: TI Spreeta (Chinowsky/Yee) • Distributed and Autonomous, Multiple Measurements: – Insertion-based probes – Compact signal processing – Streamlined, robust optical path Surface Plasmon Resonance Portable Biochemical Sensing Systems • Who are we? – Karl Booksh, Biochemistry, Arizona State University (Probes and Functionalization) – Denise Wilson, Electrical Engineering, University of Washington (Signal Processing and Systems Integration) – Are we interdisciplinary? Tight integration of biochemistry and electrical engineering • Goal of this Research – Surface Plasmon Resonance • Field monitoring at numerous locations • What defines the problem? – Ability to sense specific analytes at high sensitivity/low detection limit – With high resilience to ambient fluctuations • light, temperature, • other factors that influence bulk refractive index – In a manner that allows continuous sampling with little overhead – In a footprint that is non-intrusive or easily carried (handheld) Surface Plasmon Resonance Portable Biochemical Sensing Systems Basic Operation Sample Metal Surface Plasma Wave Evanescent Wave θinc Substrate Incident Light Optical Fiber w/ Cladding When the wave vector closely matches that of the surface plasmon at the metal-sample interface, reflected light is significantly attenuated Reflected Light Gold Coating Exposed Core ko = 2p/l Surface Plasmon Resonance Portable Biochemical Sensing Systems Configurations • Point of resonance can be detected at a – Particular angle (constant wavelengh interrogation) – Particular wavelength (constant angle interrogation) • Constant Angle – Polychromatic light source at constant angle of incidence • Constant Wavelength – Monochromatic light source at different angles of incidence Constant Angle is chosen here for: inexpensive light source, easy alignment, and simpler, more compact configuration (= less overhead) Surface Plasmon Resonance Portable Biochemical Sensing Systems Sensor Design Sampling Options: • In-line • “Dip” insertionbased probe The probe configuration is : • easily replaced, easy to use • Less prone to sensor layer blocking, but can be • more sensitive to ambient fluctuations • more susceptible to fouling Surface Plasmon Resonance Portable Biochemical Sensing Systems Typical Output Air Increasing RI Raw Data (background overwhelms resonance) Referenced Data (Resonance is evident) Surface Plasmon Resonance Portable Biochemical Sensing Systems Summary of Effort Multivariate Calibration Approach #1 (Traditional) Software High Resolution Photodetection Communication/ ADC Overhead Measurement to Reference Ratio Approach #2 (Voltage-Mode, Partially Integrated) Low Resolution Photodetection Integration Time Programming “Flatlining” Reference Ratio High Resolution Regression Multivariate Calibration Software Low Resolution Regression Surface Plasmon Resonance Portable Biochemical Sensing Systems Summary of Effort Approach #3 (Pulse-Mode, Fully Integrated) Multivariate Calibration Software Low Resolution Photodetection “Flatlining” Current Scaling Conversion to Pulse Mode Approach #4 (Current-Mode, Fully Integrated) Low Resolution Regression Multivariate Calibration Software Low Resolution Photodetection Dark Current Compensation “Flatlining” Current Scaling Low Resolution Regression Surface Plasmon Resonance Portable Biochemical Sensing Systems System-on-Chip Implementations Approach #2 All Designs are mixed signal, fabricated in standard CMOS Approach #4 6 /12... 6 /6 6 /6 6 /12... 6 /6 6 /6 4 /4 Sp_0 Sp_1 4 /4 Sp_7 Vdd 6 /9 18/6 18/6 18/6 18/6 6/6 6 /9 Vbuff 4 /4 6/6 6/12 Chold Vi Σ Precharg e ViΥ 6/12 18/6 18/6 18/6 6/6 6/6 18/6 Vi Vi Vref 6/6 Vcomp 6/6 Vbias 6/6 Approach #3 Vset Idark Mdark*Idark (a) C 15/6 Hold 6/6 6/6 6/6 6/6 Surface Plasmon Resonance Portable Biochemical Sensing Systems System-on-Chip Implementations Pixel Analog Sampling Digital Control 2mm Phototransistor 15 pixel array fabricated on a 1cm2 die in the 1.5 micron AMI process through MOSIS Surface Plasmon Resonance Portable Biochemical Sensing Systems System-on-Chip Implementations Approach SOC Integration Size (l X l ) Traditional None Big Voltage Mode Partial 200 X 1800 Pulse Mode Full 200 X 1200 Current Mode Full 200 X 1000 Current Mode Approach Traditional Voltage Mode Pulse Mode Prediction Error 6.07% 6.05% 7.8% RI Resolution 5 X 10-4 2 X 10-4 6 X 10-4 Surface Plasmon Resonance Portable Biochemical Sensing Systems • What’s the bottom line? – Benchmarking has shown system-on-chip to be competitive with software solutions – Compact, low user-overhead, low-power SPR nodes have been enabled: • Environmental Monitoring (e.g. coastal/ocean/freshwater) • Denise sensor networks for maintaining public safety (e.g. water supply) • Biomedical applications (e.g. point of care, preventative heart attack monitoring) – Students (3 MS, 2 undergraduate, 2 of which are women) – Outreach/Broader Impact • SPR modeling and simulation integrated into electronic nose toolbox • www.ee.washington.edu/research/enose – Technology Transfer • Probe design is patented and licensed to two companies in Phoenix • SOC designs are fabricated in standard CMOS • Optical components are modular and readily available Surface Plasmon Resonance Portable Biochemical Sensing Systems • Publications – Denise M. Wilson and Lisa E. Hansen, “Current-mode System-on-Chip for SPR Sensing Systems,” IEEE Sensors Journal, submitted for publication, June 2006. – Lisa E. Hansen and Denise M. Wilson, “System-on-chip Surface Plasmon Resonance Sensors Using Pulse-based Interface Circuits,” IEEE Sensors Journal, submitted for publication, March 2006. – M.W. Johnston, Lisa E. Hansen, and Denise M. Wilson, “System-on-Chip Circuit Architecture for Eliminating Interferents in Surface Plasmon Resonance Sensing Systems,” IEEE Sensors Journal, submitted for publication, January 2006. – Lisa E. Hansen, Matthew Johnston, and Denise M. Wilson, “System-on-chip Surface Plasmon Resonance Sensors Using Pulse-based Interface Circuits,” IEEE Sensors: Irvine, California, October 2005. – Matthew Johnston, Denise Wilson, Karl Booksh, and Jeffrey Cramer, “Integrated Optical Computing: System on Chip for Surface Plasmon Resonance Imaging,” Intl. Symp. Circuits and Systems, ISCAS: Kobe, Japan, May 2005. – Lisa Hansen, Matthew Johnston, and Denise Wilson, “Pulse-based Interface Circuits for SPR Sensing Systems,” Intl. Symp. Circuits and Systems, ISCAS: Kobe, Japan, May 2005. – Denise M. Wilson, Mike Warren, Karl Booksh, and Louis Obando, “Integrated Optical Computing for Portable, Real-time SPR Analysis of Environmental Pollutants,” Eurosensors 2002: Prague, Czech Republic, September 2002.