Name - Ms Brown's Chemistry Page
advertisement

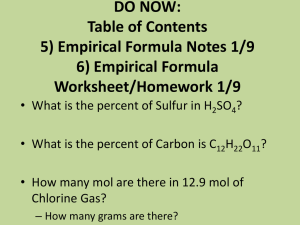
Name _________________________________________________ Date ______________ Block __________ Chemistry Review Chapter 6: The Mole Fill in the blank: Use the word bank below to answer questions 1 - 5 Empirical formula Molar mass Formula unit Average atomic mass Avogadros constant Percentage composition One mole 1. A formula expressing the simplest ratio of cations to anions in an ionic compound is known as a(n) _____________________________________. 2. The number of particles in 1 mol of a substance is known as ______________________________. 3. ________________________ is the mass in grams of 1 mol of a substance. 4. The simplest ratio among the elements represents the compounds ___________________________. 5. ______________________ represents the number of atoms in exactly 12 g of carbon – 12 Practice Problems (Show your work!!) 6. How many moles of copper are in a penny containing 1.8 x 1021 atoms? 7. Assume that you have 2.59 mol of aluminum foil. How many atoms of aluminum do you have? 8. A neon sign contains 4.5 x 10-2 mol of neon. How many grams of neon are in this sign? 9. How many grams are in the 2 mol of phosphorous used to coat your television screen? 10. How many grams are present in 7.2 mol of table salt? 11. One cup of whole milk contains 290 mg of calcium. How many moles of calcium are in the milk? 12. An aspirin contains 325 mg of acetylsalicylic acid (C9H8O4). How many moles of acetylsalicylic acid are in each tablet? 13. Write the correct chemical formula, calculate the molar mass and the percent composition for each compound: Name of compound Chemical formula Molar mass % composition Zinc carbonate Iron (III) oxide Ammonium sulfate Potassium permanganate Calcium acetate 14. Magnetite is an iron ore with natural magnetic properties. It contains 72% iron and 28% oxygen. What is its empirical formula? 15. An oxide of arsenic contains 3.26 g of arsenic and 1.04 g of oxygen. What is the empirical formula for this oxide? 16. Chemical analysis of a 10 g sample of oil of wintergreen shows that it contains 6.321 g of carbon, 0.53 g of hydrogen and 3.16 g of oxygen. What is the empirical formula for oil of wintergreen? 17. An acid is analyzed in the laboratory and the following results are obtained: 3.1 % hydrogen, 31.6 % phosphorous, 65.3 % oxygen. It has a molar mass of 98 g/mol. What is the molecular formula for this acid? 18. A sample is analyzed and is found to contain the following percent composition of elements: 26.6% potassium, 35.4 % chromium and 38.0% oxygen. What is the empirical formula for this compound? 19. A compound was analyzed and found to have the empirical formula CH. It was also determined to have a molar mass of 78 g/mol. What is the molecular formula of this compound? 20. What is the formula for a hydrate which consists of 90.7 % SrC2O4 and 9.30 % H2O? 21. What is the formula of a hydrate that is 86.7% Mo2S5 and 13.3% H2O? 22. During lab, 1.62 g of CoCl2 * X H2O were heated. After heating, only 0.88 g of CoCl2 remained. What was the formula of the original hydrate? 23. Determine the percent of WATER in K2S · 5 H2O.