Biomolecules
advertisement

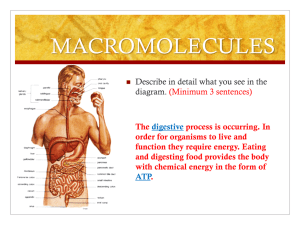
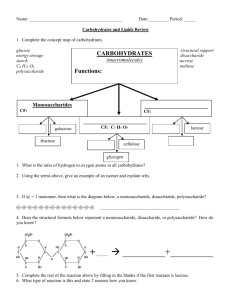
Bellringer Describe in detail what you see in the diagram. (Minimum 3 sentences) The digestive process is occurring. In order for organisms to live and function they require energy. Eating and digesting food provides the body with chemical energy in the form of ATP. Schedule: Today you will be able to: Compare the structures & functions of different types of biomolecules, including carbohydrates, lipids, proteins, and nucleic acids. SE 9A Pre-test Biomolecule Hmwk Review IAN 11 Biomolecule graphic organizer IAN 12 Construct glucose molecule Biomolecules You are what you eat! Agenda 1. Carbohydrates 2. Proteins 3. Lipids 4. Comparing biomolecues 5. Constructing glucose molecules Ms. Jackson’s Lunch Agenda 1. Carbohydrates 2. Proteins 3. Lipids 4. Comparing the biomolecules 5. McMush Lab Carbohydrates aka… Sugar Carbs Starch How does our body break down this bread? Breaks down into microscopic molecules Loaf of bread Monosaccharide Bread crumbs Polysaccharide Disaccharide Structure Many Sugar Polysaccharide Structure Two Sugar Di saccharide Structure One Sugar Monosaccharide Function Provide QUICK energy to the body! Bellchallenge: Loaf of bread Monosaccharide Explain what you think is happening here. (at least 3 sentences) Bread crumbs Polysaccharide Disaccharide The diagram shows a picture of bread (starch). It also maps out the break down of carbohydrates from their most complex form (polysaccharide) to their least complex (monosaccharide). This process is called hydrolysis. Agenda 1. Bellchallenge: Carbohydrates 2. Carbohydrate/lipid Homework 3. Lipids 4. Test for Organic Compounds (Part A, C & D) 5. IAN Check (TOC & word wall) 6. Alternate assignment: Pp. 48, 39, 43 Breaks down into microscopic molecules Fats, oils, waxes, steroids Monomer: 3 fatty acids + glycerol Polar head (&hormones) Non-Polar head Phospholipids & steroids join together w/proteins in the cell membrane Function Made mainly of carbon and hydrogen (few oxygen) Fat best method of STORING forms cell membranes Insulates nerve cells (myelin) Insulates body (maintains homeostasis) How are complex carbohydrates formed and broken down? Dehydration Synthesis Combining single compounds into a complex one by removing water monosaccharide + monosaccharide ----> disaccharide + water C6H12O6 + C6H12O6 ----> C12H22O11 + H2O (Process forms disaccharides & polysaccharides) Hydrolysis Addition of WATER to a compound SPLITS it disaccharide + H2O ---> monosaccharide + monosaccharide C12H22O11 + H2O ---> C6H12O6 + C6H12O6 Ms. Jackson’s Lunch Bellchallenge: Describe what you see (at least 3 sentences) This is an amino acid. It is the monomer for a protein. It contains C, H, O and N. It has 3 groups: an amino group, an R-group, and a carboxyl group. The R-group is considered a variant group because it changes. Friday 9/17/10 Agenda 1. Proteins 2. Test for Organic Substances (Parts C,D &E) 3. Complete analysis questions 4. HW: Venn Diagramcarbohydrates, lipids, proteins Proteins aka…. Whey Protein Meat Polypeptide Peanut butter Breaks down into microscopic molecules Polypeptide Meat Amino Acid Monomer: amino acid Polymer: polypeptide Monomer: Amino Acids •Contains C, H, O, N •20 types •Has both hydrophobic & hydrophylic ends •Differ in R-group •R-group can be acidic, basic or neutral •Makes polypeptide then makes proteins Polymer: Polypeptide (peptide means bond) •Formed by dehydration synthesis •Sequence determined by DNA •3-D and folds to take up less space Function of Proteins Provides us with building blocks for life! Also regulate most functions in a cell. Glycoproteins (antigens) Combines w/DNA to form chromosomes Turns genes on and off Antibodies (fights disease) Function of Proteins Provides structure & strength (fibers) Transports molecules in & out cells Hemoglobin (transports O2) Enzymes (speeds up rxns)has –ase suffix Acts as hormones (insulin)many proteins have suffix of -in Bellringer: Describe in detail what you see in the diagram. (minimum 3 sentences) This is a picture of ATP. ATP is one type of polymer of a nucleic acid. It is made of adenine, sugar, and 3 phosphate groups. Monday 9/20/10 Agenda 1. Nucleic Acids 2. FinishTest for Organic Substances (Parts C,D &E) 3. Complete analysis questions 4. HW due Friday: Venn Diagram- carbohydrates, lipids, proteins & nucleic acids Nucleic Acids Contains C, H, O, N, P Monomer: NUCLEOTIDES Nucleotides consist of 3 parts: 1. 5-Carbon Sugar 2. Phosphate Group 3. Nitrogenous Base Nucleotide: Nitrogen Bases In DNA: C-G A-T In RNA: C-G A-U 5 types Cytosine Guanine Adenine Thymine (in DNA only) Uracil (in RNA only) Purines or pyrimidines Nucleotides: 5-carbon sugar and phosphate group deoxyribose 2 types of sugars Ribose (in RNA only) Deoxyribose (in DNA only) Phosphate group Contains phosphorus & oxygen Polymer: polynucleotide ribose Function Polypeptide: DNA (deoxyribonucleic acid) contains the genetic code stores & transmit heredity/genetic information found in the nucleus (mitochondria) Double helix) stranded (double Function Polypeptide: RNA (ribonucleic acid) Carries info from DNA to cell Helps in protein synthesis found in ribosomes & nucleoli Single stranded Polypeptide: ATP Contains adenine, ribose sugar, 3 phosphates Stores and releases energy Concept Map Section 2-3 Carbon Compounds include that consist of that consist of that consist of that consist of which contain which contain which contain which contain Concept Map Section 2-3 Carbon Compounds include Carbohydrates Lipids Nucleic acids Proteins that consist of that consist of that consist of that consist of Sugars and starches Fats and oils Nucleotides Amino Acids which contain which contain Carbon, hydrogen, oxygen Carbon, hydrogen, oxygen which contain which contain Carbon,hydrogen, oxygen, nitrogen, phosphorus Carbon, hydrogen,oxygen, nitrogen, Which biomolecule has the most energy!? C-H bonds Count the number of C-H bonds in your monosaccharide picture. Count the number of C-H bonds in your saturated or unsaturated bond picture Which biomolecule (carbs or fats) have more C-H bonds? The number of C-H bonds = the amount of energy The more C-H bonds a biomolecule has, the more energy it has! Fats have the most energy because they have the most C-H bonds! Video clips: Burning Marshmallow Oil lamp Cooking Oil Car Time for TEAM CHALLENGE! Which popular plant process forms glucose? Photosynthesis Which elements form a glucose molecule? Carbon, hydrogen, oxygen What is the molecular formula for glucose? Each group will create a polymer using dehydration synthesis process. C6H12O6 Biomolecule Matchup McMush Lab