Lecture 2 - iowacellbiologyspring2011
advertisement

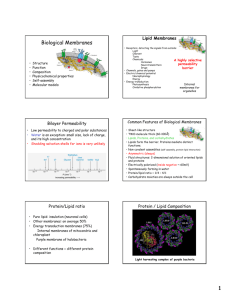
Lecture 2 1. Membrane functions a. Compartmentalization i. Type of membranes 1. Plasma membrane a. Encloses contents of the entire cell 2. Nuclear and Cytoplasmic Membranes a. Enclose diverse intracellular spaces ii. Isolate 1. Allows for internal processes to occur with out external interference 2. Allows for cellular activities to be regulated independently of one another b. Scaffold for biochemical activities i. Extensive Framework 1. Allows components of the cells to be arranged in a fashion that allows for effective interaction. c. Providing a selectively permeable barrier i. Communication 1. Exchange molecules between compartments (selectively) ii. Prevent unrestricted exchange of molecules d. Transporting solutes i. Active Transport 1. Allows for sugars and amino acids to build up a. Required to fuel the metabolism b. Required to build the body’s macromolecules 2. Establish Ion gradients a. Especially important for nerve and muscle cells e. Responding to External Signals i. Signal transduction 1. Response of the cell to external stimuli 2. Contains receptors that bind with specific ligands a. Could possibly result in a signal +/- internal f. Intercellular Interaction i. Plasma membrane 1. Regulates the interaction of the cell and its neighbors. 2. Allows for cell recognition and signaling a. Required for info and material exchange between cells. g. Energy Transduction i. Energy conversion 1. Photosynthesis a. Energy in sunlight absorbed by membrane bound pigments b. Converted into chemical energy i. Stored in carbs 2. Carbs and Fats a. Allows for conversion into ATP ii. Occurs commonly w/in membranes of chloroplasts and mitochondria 2. History a. Karl von Nagel and Pfeffer (1855) i. Plasma membrane b. Overton (1899) i. The more oily the solute, the faster it moves/recovers ii. Experiment 1. Osmosis in root hair cells a. Rate of water entry=rate of return to full volume b. Sucrose solution i. Water lower in concentration ii. Water leaves via osmosis 2. Correlated lipid solubility and the entry of substances into the cells. a. The more lipid soluble the solute, the faster it enters the cell b. Cell surface consists of lipids c. Langmuir (1917) i. His trough measured lipid area ii. Created trough with movable barrier 1. Allowed to measure lipid area iii. Observed that lipids at air/water interface form a coherent layer d. Gorter and Grendel (1925) i. Discovered lipid bilayer ii. Red blood cells have lipid bi-layer 1. Extracted lipids from RBCs a. Monolayer of lipids covered twice the amount of area as calculated i. Therefore, lipid bi-layer is two layers thick e. Davson and Danielli (1935) i. There is protein on the bilayer ii. There is a difference between membranes of artificial membranes’ and real cell membranes’ surface area 1. Artificial doesn’t have protein f. Robertson (1927) i. Unit membrane ii. Invention of electron microscope 1. Able to see that membranes have trilaminar appearance with 2 dense lines g. Singer and Nicholson (1972) i. Fluid mosaic Model of membrane 1. Differed from previous models because it was a mosaic 2. Dynamic and Fluid 3. Cell Biology Technique a. Electron Microscopy i. Uses electrons instead of light to form images ii. Electron beam from a tungsten filament is accelerated by high voltage iii. Beam is focused with magnetic field iv. Electrons pass through the specimen and strikes a fluorescent screen 1. Differential scattering by electrons creates image 2. Electron scattering proportional to the mass thickness of that part of the specimen. b. Preparing Specimen for Transmission EM i. Fix tissue with chemicals 1. Ex: Glutaraldehyde ii. Dehydrate tissue 1. Ex: Alcohol iii. Embed tissue in plastic 1. Ex: Epon. Araldite, epoxy iv. Cut thin sections with ultramicrotome 1. 70nm sections v. Stain tissue with heavy metals 1. Os, Pb, W, or U a. They scatter electrons and generate contrast vi. Examine sections in vacuo 1. They have to be in a vacuum c. Preparing Specimen for Freeze Fracture EM i. Frozen tissue is fractured with a knife 1. Crystals are bad a. Caused from over freezing ii. A heavy metal layer is deposited on a fractured surface 1. Au or Pt used iii. A thick layer of Carbon is deposited, forming a cast (replica). iv. The metal-carbon replica is viewed in the TEM