Rate Law and Activation Energy Methyl Blue
advertisement
Rate Law and Activation Energy
Methyl Blue
Determining the Rate Law using the
Time Dependent Rate
The Reaction
The Reaction
MB + (aq,violet) + OH - (aq) ® MBOH (aq,colorless)
ratet = -
D éë MB + ùû
Dt
x
y
= k éë MB ùût éëOH ùût = Ae
+
-
-
Ea
RT
x
éë MB + ùût éëOH - ùût
y
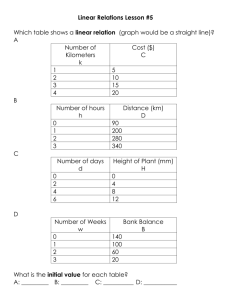
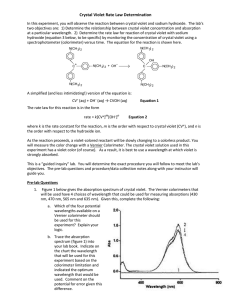
• Reaction takes about 30 s to a few minutes depending on the initial concentrations
• Can use a continuous monitoring method
• Because there is a color change we can use spectroscopy
The Reaction
MB+ (aq,violet) + OH - (aq) ® MBOH(aq,colorless)
• In this experiment, the initial concentration of the hydroxide is at least 1000 times
larger than the concentration of the MB
y
• This means that éOH - ù » éOH - ù
ë
ût ë
û0
• And
ratet = -
D éë MB + ùû
Dt
where
{
k ' = k éëOH - ùû 0
y
}
{
y
» k éëOH - ùû 0
y
}
x
éë MB + ùût = k ' éë MB + ùût
x
Figuring out x
• To figure out the order with respect to the MB+ we will
observe [MB+] vs t
• To do this we use Beer’s Law
At = e b éë MB+ ùût
Absorbance
Constants
• Plot At vs t, LN(At) vs t and 1/At vs t to see if the reaction is
zeroth order, first order or second order wrt MB+
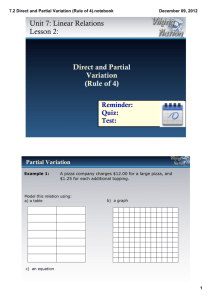
Figuring out x
At
LN(At)
If straight x = 0
Which one gives a straight line?
1/At
If straight x = 1
If straight x = 2
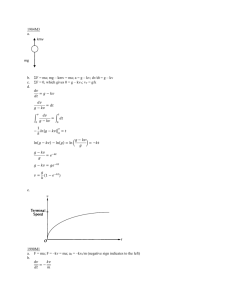
The Math
First Order
Zeroth Order
éë MB ùût = k 't - éë MB ùû 0
LN éë MB + ùût = k 't - LN éë MB + ùû 0
At
A
= k 't - 0
eb
eb
æAö
æA ö
LN ç t ÷ = k 't - LN ç 0 ÷
è eb ø
è eb ø
At = e bk 't - A0
LN ( At ) - LN ( e b ) = k 't - LN ( A0 ) - LN ( e b )
+
+
• The slope of the linear curve will
get you k’=k[OH-]oy to within a
constant
• Repeating the experiment at a
second [OH-] will get you access to
how slope depends on [OH-] and
get y
Second Order
1 / éë MB + ùût = k 't + 1 / éë MB + ùû 0
e b / At = k 't + e b / A0
1 / At =
k'
t + 1 / A0
eb