Loading control
advertisement

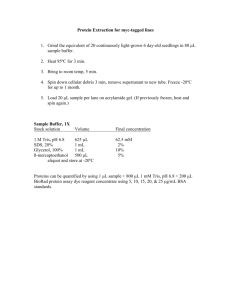
Western in A Day Ning Tingting, Ph.D Field Application Specialist, BIO-RAD Tuesday, March 22, 2016 印记法 Northern Blot RNA Protein DNA Western Blot Far Western Blot Dot Blot Southern Blot Transfer Methods The principle of Electrophoretic transfer Proteins migrate to the membrane following a current (I) that is generated by applying a voltage (V) across the electrodes, following Ohm’s law: V=IxR Transfer Methods The principle of Electrophoretic transfer 1. the applied voltage and the distance between the electrodes play a major role in governing the rate of elution of the proteins from the gel. 2. the size, shape, and charge of the protein and the pH, viscosity, and ionic strength of the transfer buffer and gel %T influence the elution of particular proteins from gels There are practical limits on field strength, however, due to the production of heat during transfer. Joule heating is proportional to P = I x V = I2 x R Transfer Methods The principle of Electrophoretic transfer Joule heating is proportional to P = I x V = I2 x R Joule heating • Buffer resistance • Buffer buffering capacity • Inconsistent field strength and transfer • Gel to deteriorate and stick to the membrane The major limitation of any electrophoretic transfer method is the ability of the chamber to dissipate heat Transfer Methods Types of Electrophoretic transfer Tank Blotting Semi-Dry Blotting Flexibility Flexible voltage settings, blotting times, and cooling requirements; flexible electrode positions (Trans-Blot and Trans-Blot Plus cells) (Trans-Blot and Trans-Blot Plus cells) without cooling Quantitative vs qualitative results Quantitative transfer of low molecular weight proteins possible under conditions that allow efficient binding to the membrane Some low molecular weight molecules will be transferred through the membrane without binding quantitatively Molecular weight range Broad molecular weight range Variable transfer efficiencies for proteins >120 kD (may be improved with discontinuous buffer system); low molecular weight proteins may be transferred through membrane Transfer time Extended transfer (up to 24 hr) possible without buffer depletion; rapid transfers (15–60 min) obtained under high-intensity conditions Rapid transfers; extended transfers not possible due to buffer depletion Temperature control Specific temperature regulation with cooling coil and refrigerated water recirculator; permits transfers at low temperatures (4–10°C), for example, native enzyme transfers Temperature regulation by external cooling is not possible Buffer capacity Up to 10–12 L (Trans-Blot Plus cell) or as little as 450 ml (Mini Trans-Blot cell); length of blotting time not restricted by limited buffer capacity Minimal, ~250 ml per experiment; reduced cost of reagents and experiment time Membrane, Buffer & Power conditions Membrane selection Membrane Nitrocellulose Supported Nitrocellulose Pore size 0.45 µm 0.2 µm 0.45 µm 0.2 µm Binding capacity Comments (µg/cm2) 80-100 good general purpose blotting membrane 80-100 Pure nitrocellulose cast on an inert synthetic support; increased strength for easier handling and for reprobing Sequi Blot PVDF 0.2 µm 170-200 High mechanical strength and chemical stability; high binding capacity, also with SDS used for protein sequencing of even low abundance sample Immun Blot PVDF 0.2 µm 150-160 High mechanical strength and chemical stability; high binding capacity, also with SDS used for protein immunodetection ! PVDF membrane must be wetted in 100% methanol prior to use but may be used with a transfer buffer that contains no methanol Membrane, Buffer & Power conditions Transfer buffer selection General recommandation: SDS in buffer: protein mobility binding efficiency to membrane – SDS confers a negative charge to positive and neutral proteins – increases transfer efficiency of large proteins – recommended when using SDS: PVDF or positively charged nylon membranes – will increase the relative current, power and heating – may affect the antigenicity of some proteins – à discontinuous system with only 0.01 % SDS Membrane, Buffer & Power conditions Transfer buffer selection General recommandation: Methanol in buffer: protein transfer from gel binding to nitrocellulose – not necessary with PVDF or nylon membranes – gel shrinks, the pores of the gel are reduced – removes SDS from SDS-protein complexes – precipitation, denaturing, loss of biologic activity Membrane, Buffer & Power conditions Transfer buffer selection Standard Towbin buffer contains 25 mM Tris, pH 8.3, 192 mM glycine, 20% (v/v) methanol and, occasionally, 0.025–0.1% (w/v) SDS. A buffer similar in composition to the standard Towbin buffer is the Bjerrum and Schafer-Nielsen buffer (48 mM Tris, pH 9.2, 39 mM glycine, 20% methanol), which was developed for use in semi-dry applications CAPS buffers (10 mM CAPS, pH 11, 10% methanol) may be preferable for transfers of high molecular weight proteins (for example, >150 kD) and in cases where the glycine component of Towbin buffer may interfere with downstream protein sequencing applications Dunn carbonate buffer (10 mM NaHCO3, 3 mM Na2CO3, pH 9.9, 20% methanol) may produce higher efficiency transfers and improve the ability of antibodies to recognize and bind to proteins Membrane, Buffer & Power conditions Transfer buffer selection Discontinuous Tris-CAPS Buffer System (Semi-Dry Transfers) 60 mM Tris, 40 mM CAPS, pH 9.6, plus 0.1% SDS 60 mM Tris, 40 mM CAPS, pH 9.6, plus 15% methanol Membrane, Buffer & Power conditions Power conditions for Electrophoretic Transfers Selecting power supply settings During tansfer, the resistance decreases as a result of Joule heating, except for semi dry blotting where R increases due to ion depletion. Transfer under constant voltage U cst = R x I -need a cooling system - field strength will remain constant, providing the most efficient transfer possible Transfer under constant current U = R x I cst -heating minimized - proteins will be transferred more slowly due to decreased field strength Transfer under constant power P cst = R x I2 = U2 / R -current increases but less than for Vcst (√), decreased field strength - alternative to constant current for regulating heat production during transfer Performing the transfer Tips •Remove all air bubbles at each step with a roller •To avoid ghost prints and other artifacts, do not move the membrane and/or gel after it is positioned •Place the transfer tank onto a magnetic stirplate •The tanks are effective thermal insulators and limit the efficient dissipation of heat. Therefore, placing blotting cells in a coldroom is not an adequate means of controlling transfer buffer temperature. Use the internal cooling devices •For SD, use one extra thick filter paper instead of two or three thin or thick filter paper to avoid air bubbles between them •For SD, the membrane and filter paper should be cut to the same size as the gel Detection Detection Detection Methods Detection Immunological Detection Procedure Detection Immunological detection methods A’. Colorimetric amplified AP detection What is important to a western blot experiment? • Can I see my target proteins? – if not, what’s wrong? • Transfer • Sample • Detection sensitivity – if yes, how reliable? • Accurate? • Reproducible? • Can I get away from the tedious work? “Bio-Rad V3 WB workflow” to address these issues • Can I see my target proteins? – if not, what’s wrong? • Transfer (Verify better monitoring of transfer efficiency) • Sample (Visualize sample quality control) • Detection sensitivity (more sensitive detection reagents) – if yes, how reliable? • Accurate? (Validate – Quantification and loading control) • Reproducible? (transparency of the whole process for better monitoring, such as loading error, uneven transfer etc.) • Can I get away from the tedious work? Fast procedure Multiplexing Content • What is “Bio-Rad V3 Western Blot Workflow” • What is important to a western blot experiment? • Visualize gel run • Verify Transfer efficiency • Validate quantitative analysis • Multiplexing of the detection • Comparison between Bio-Rad “Bio-Rad V3 Western Blot Workflow” and the traditional western blot approach What is it? Bio-Rad V3 Western Blot workflow is a Bio-Rad complete western blot solution incorporated with new technologies and products, such as TGX stain free gels, Trans-blot Turbo and ChemiDoc MP imager system, to provide a best practice in western blot experiment to ensure higher confidence in the experimental findings over the traditional western blot workflow. The traditional western blot workflow Traditional WB Approach Gel prep >1h By ChemiDoc MP (optional view under UV) ~1h Electrophoresis 1-3 h blotting Immunodetection ~5h Imaging & data analysis >30min Often need reprobing ~5h By eye Loading control reprobing Visualize 眼见为实 Bio-Rad Stain Free Technology ChemiDoc MP system Stain Free Pre-cast Gels Stain Free Technology 1. Stain-free compound is premixed with the gel, therefore no staining step required 2. Gel image can be generated 5 minutes after electrophoresis is finished 3. UV induced fluorescent gel or blot image 4. Low background produced and staining is uniform across the gel and blot 5. Non-reversible staining on both the gel and blot (can not wash away) 6. The same gel is used for both total protein loading control and transfer for western blotting Sample quality control: Stain Free Technology to Visualize the gels within 5 min after gel run Fraction # 1 2 3 4 C C A A C C A A C C A A C C A A T.P. 75 kDa 50 kDa 25 kDa A total protein gel image allows a double check of the protein profiles in each sample to avoid artifacts caused by inaccurate protein assay and/or protein degradations. The sample quality control tells you when to terminate your experiment… Don’t waste time and money on bad samples Verify 确认转印 Fast Blotting of TGX Stain Free gels make it easy to verify transfer efficiency 3 Min Trans-Blot Turbo with TGX gels 15 Min Tank Blotting with TGX gels How do I know transfer was successful and how to optimize it? 1. Traditional approach • only indicative, prestained protein standards • no image recording, not quantitatively 2. Bio-Rad V3 WB approach • quantitative Stain Free Gel image • true visualization of the protein samples 3. To optimize transfer conditions 1. easy and fast 2. If some proteins remained in the gel after transfer, set up another blotting with the same gel to give another 5 min of transfer 3. Add this extra time to the original setting for next blotting experiment Now you can see proteins on the gels before transfer Stain free image of protein samples on the Gel Double check your loading consistency Double check the sample quality Now you can see proteins on the post-transfer gels Stain free image of a Post-Blot Gel No more guess about the transfer efficiency. Now you have the control – you can calculate how many percent of the total protein are still left in the post-transfer gel… Now you can see proteins on the blot Stain Free image of proteins on a blot Worry about protein loss during striping for reprobing? No more worries, now you have the control – simply put the blot in the ChemiDoc MP to take a quick look… Validation 定量校验 Linear dynamic ranges Loading control Western Blot can be more quantitative Introduction to Western Blot Validation Validation of western blot results: Why and How? How? Typically, a specific housekeeping protein is used as loading control (GAPDH, Actin, Tubulin…) Experimental errors are identified by quantitative differences in HKP Target protein data is normalized to the HKP signal Most customers do chemiluminescence detection → Strip and reprobe or cut the membrane Sounds easy, right? What‘s the problem with both experiments below? Experiment B (same publication!) Experiment A What is loading control? What are the commonly used loading control proteins? Samples of loading control in publication -PNAS Fig. 7. Western blots showing the extents of calmodulin over-expression in DG75 cells (A) and splenic B-cells (B). The levels of calmodulin in nuclear and cytoplasmic extracts of calmodulin over-expression experiments with and without antiIgM treatment in DG75 cells and in splenic B-cells (Figs. 2A and 5G, respectively) were determined by Western blot analysis. The Western blots were performed using the WesternBreeze immunodetection system (Invitrogen) according to the manufacturer's instructions, using calmodulin antibody (FL-149; Santa Cruz Biotechnology). Tubulin antibody (clone B-5-1-2; Sigma-Aldrich) was used as loading control for cytoplasmic extracts and histone H1 antibody (FL-219; Santa Cruz Biotechnology) as loading control for nuclear extracts. What’s wrong? What’s wrong? What’s wrong? Western Blotting Help!!!! - Problem with an internal control (Jul/28/2004 ) Hello ALL! I’m hoping someone can explain what is going on with some of my data!!!! We are currently checking cell viability post cell explant from saphenous vein tissue. Our viability data indicates that we are isolating less cells from the tissue as time increases and that these cells in culture are less viable. So to look at specific cell markers we thought it locgical to run our samples on a SDS-PAGE and use Beta Actin as a loading control. Time after time we are loading equal amounts of protein per lane but our cell specific marker is decreasing (which is logical...that is the amount of cells expressing H-caldesmon.....) but our B-actin which should be ubiquitous to all cells is also decreasing at a similar ratio. This doesnt make sense to me as we were hoping to see loss of protein cell-specific protein, only!! We have used BCA to measure our protein concentations in our cell lysates, and have used kits from different manufacturers with similar results. We have used different antibodies, both monoclonal and polclonal. We have also used different H-caldesmon antibodies. Each time with similar results. Could cell death (which we know by our viability experiments) contribute to the decrease in beta-actin (our internal control) despite the loading of equal amounts of protein. By the way, this has also been done with GAPDH and tubulin. I'm at a loss and cant explain this. PLEASE HELP?!!! Doubt ELECTROPHORESIS Volume 27, Issue 14, pages 2844–2845, No. 14 July 2006 β-Actin is not a reliable loading control in Western blot analysis Angela Dittmer, Jürgen Dittmer Dr. Abstract β-Actin is often used as a loading control in Western blot analysis. We analyzed the ability of β-actin-specific antibodies to recognize differences in protein loading. We found that, at higher total protein loads as required for the detection of low-abundance proteins, β-actinspecific antibodies failed to distinguish differences in actin protein levels. Diluting the antibody working solution or changing the incubation time had little effect on this phenomenon. This shows that β-Actin is not a reliable loading control in Western blot analysis. In general, it appeared that, at longer incubation times, antibodies seem to be less able to pick up differences in the level of its target protein. Doubt β-actin varies in some situations • Varying expression level of β-actin in response to biomedical stimuli • Altered expression of β-actin during growth and differentiation • Changed expression of β-actin in some diseases β-actin varies in some situations Expression difference @PCR level Reason 2 – overexposure In the case of the polyclonal anti-b-actin antibody, no change in band intensity could be observed in the range of 7.5–1.88 mg of total protein. At 0.94 mg protein the signal gradually decreased and was undectable at 0.12 mg or lower. The monoclonal anti-b-actin antibody generated a similar picture, except that similar band intensities were found in a broader range of protein loads between 0.47 and 7.5 mg. What are the alternatives for loading control? What are the alternatives for loading control? What are the alternatives for loading control? Option 1- Irreversible • • • • Coomassie staining Silver staining Amido black staining Fluoresce staining Option 2 - reversible Analytical Biochemistry Volume 401, Issue 2, 15 June 2010, Pages 318-320 Reversible Ponceau staining as a loading control alternative to actin in Western blots Isabel Romero-Calvoa, Borja Ocóna, Patricia Martínez-Moyaa, María Dolores Suáreza, Antonio Zarzueloa, Olga Martínez-Augustina and Fermín Sánchez de Medina , a, a Departments of Biochemistry and Molecular Biology II and Pharmacology, School of Pharmacy, University of Granada, Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), Campus de Cartuja s/n, 18071 Granada, Spain Abstract It is becoming standard practice to measure a housekeeping gene, typically actin, in Western blots, as it is the rule in RNA blots. We have applied reversible Ponceau staining to check equal loading of gels and measured actin in parallel under different conditions. Our results show that densitometric analysis is comparable with both techniques. Therefore, routine quantitation of Ponceau staining before antibody probing is validated as an alternative to actin blotting. • Loss of target, Waste of membrane and time How to measure total protein local on a stain free image 1. Acquire a stain free gel image 2. Using the Image Lab software “Volume Tools” to define a volume area to cover the whole lane of a sample 3. Copy and paste the defined the area to cover other sample lanes 4. The total protein amount is measured by the “Adj. Vol.” in the data analysis table 1 2 3 4 5 6 7 Bio-Rad solution to a more reliable loading control TGX Stain Free Precast Gels & ChemiDoc MP Imager System provide The best way to measure total proteins for loading controls in a western blot experiment ChemiDoc MP system Stain Free Pre-cast Gels Benefits of the “Bio-Rad V3 WB Workflow” over traditional approaches Faster and More Reliable WB workflow Traditional WB Approach Gel prep Precast TGX stain free gel >1h ~1h 15-20 min Electrophoresis Stain Free Imaging 1-3 h ChemiDoc MP blotting Turbo More reliable loading control 3-15 min Sample quality control Immunodetection ~5h ~5h Transfer optimization Imaging & data analysis ChemiDoc MP <30min >30min Protein visualization on blot Multiplex WB Often need reprobing ~5h Loading control reprobing No need to reprobe Multiplexing WB to analyze phosphoproteins Nature Methods 2, 79 - 81 (2005) Western blot analysis with quantum dot fluorescence technology: a sensitive and quantitative method for multiplexed proteomics Western blot images of p42 MAPK (a, green) and phosphorylated p42 MAPK (b, red) expression in serum-starved 3T3 cells in response to PDGF stimulation. Cells were harvested at 0, 10, 20, 40 and 60 min after PDGF administration (lanes 1–5, respectively). The anti-pan MAPK primary antibody (a) was detected with Qdot 605 nm Conjugate, a red-orange dot that was pseudocolored green and the phospho-MAPK primary antibody (b) was detected with Qdot 705 nm Conjugate and pseudocolored red. (c) Overlay of images in a and b. Multiplexing WB to detect 3 different targets on the same blot using Qdot Conjugates Excitation: 415nm /100nm filter Green : Anti-GST , 565nm Red: Anti-GST-HA, (rabbit) , 605nm, Blue: Anti-GST-cMyc (mouse) 705nm Multiplexing WB to detect 4 different targets on the same blot Hsc70 (green), beta-actin (red) and ERX1 and ERX2 (blue) Titration of lac operator DNA with lac repressor protein. Increasing amounts of lac repressor protein were mixed with 40 ng of lac operator DNA, incubated for 20 minutes and then separated on a 6% nondenaturing PAGE. The gel was stained with SYBR® Green EMSA stain (green) followed by SYPRO® Ruby EMSA stain (red), components of the Electrophoretic Mobility-Shift Assay Kit . After each staining, the image was documented using an FLA-3000 laser-based scanner (Fuji) and the digital images pseudocolored and overlaid. Yellow bands indicate areas stained with both stains. This fluorescent western blot shows simultaneous detection of unphosphorylated and phosphorylated Akt1 present in serum starved and PDGF stimulated NIH/3T3 whole cell lysates. Lane 1, unstimulated NIH/3T3 lysates contain inactive unphosphorylated Akt1, green band. Lane 2, PDGF stimulated NIH/3T3 lysate contains both inactive (green band) and activated phosphorylated Akt1 (red band). Both lanes were probed with rabbit anti-Akt (pan) and mouse anti-Akt pS473 specific antibodies followed by detection with DyLight™ 549 conjugated anti-rabbit IgG (green) and DyLight™ 649 conjugated anti-mouse IgG (red) secondary antibodies. The “Bio-Rad V3 WB workflow” • Can I see my target proteins? – if not, what’s wrong? • Better monitoring of transfer efficiency – stain free gels and ChemiDoc MP imager • Sample quality control – stain free gels and ChemiDoc MP imager • More sensitive detection reagents –ChemiDoc MP imager and WesternC Chemiluminescence kit – if yes, how reliable? • Loading control – stain free gels and ChemiDoc MP imager • transparency of the whole process for better monitoring – ChemiDoc MP imager, stain free gels and low fluorescence PVDF membrane • Can I get away from the tedious work? (fast) – TGX gels and Trans Blot Turbo – Fluorescent multiplexing on ChemiDoc MP imager 联系方式: 宁婷婷 Email:Tingting_ning@bio-rad.com 电话:027-83806255-805 移动电话:18627760323