Practice Based Research
advertisement

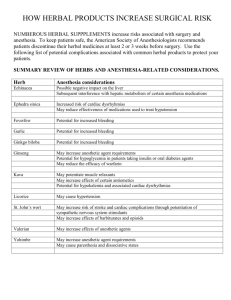
Practice Based Research at Pharmacy Practice Research Specialty Practice Unique Role and Opportunity Research Completed in a Hospital Medical / Clinical & Pharmacy Service Related Research Examples of Some Research to demonstrate the breadth of the work and the degree of opportunity & possibility Specialty Practice Site in Pharmacy Practice Based Research in Hospital Pharmacy Each Profession must complete research to advance Research improves the knowledge base & credibility and is a method of improving a professions ability to stay current. Pharmacy leaders are very “knowledgeable” in particular areas Cardiovascular Specialty – CPR/ICU/Care Teams Ambulatory Care Anticoagulation Programs Infectious Diseases Rise to that level of recognition through experience and reading/reviewing current literature (research) related to their area of practice. But who does the research…? Most Pharmacy Research is completed by pharmacists who are Pharm D or MSc trained some with residencies and some with post-PharmD fellowships Many work in a specialty environments Cardiovascular Specialty – CPR/ICU/Care Teams Ambulatory Care Anticoagulation Programs Infectious Diseases But … how do you get there? PHARMACY DEPARTMENT with Critical Mass with Common Interest Ability to Complete Research Administrative Support Time Sunnybrook in the 70’s Director: Associate Director: Jim Mann Bill Wilson Coordinators: John Iazzetta Bill Bartle Tom Paton Residency Graduate Studies @ UofT: Dr. Thiessen Kinetics Return to Sunnybrook Bench space grew to an 800 ft2 Quality Control Laboratory in early 80’s 2 Research Assistants / laboratory technicians Laboratory supplies, etc Annual operating expenditure ~ 100K + Current Resources About 1500 ft2 in K-Basement Lab Equipment: 1 LC – MS-MS; 2 LC - UV / Fl / EC Freezers, 1 – 80C; 1 -20C 3 computers in the lab plus 3 other computers Research at Sunnybrook Most Work has had an Analytical Requirement IV additive Service Pharmacokinetics Statistics Bioequivalence Drug Stability Drug-Drug-Interactions Drug Compatibility Pharmacodynamic Studies Tyramine Content of Food Herbal Content/Label Claim All studies have been completed through collaboration in fact for most … the idea/the need was generated by someone else a pharmacist … a physician … someone with a practical question Bench Research Often at a University Funding is often for a program multiple years Several types of Research * Clinical Research * Generally at a Hospital Funding is for a specific single project which may take several months or several years Survey Research Can be done anywhere Funding is often for a specific study These are questions which relate directly to patient care. The questions result in single studies that answer a specific question. The results can be used immediately in a patient There are numerous clinical questions for which there is no good data. If you are a pharmacist and you are asked such a question …where do you turn? ? In an HIV patient can we improve response to drug therapy if we make sure that drug concentrations are within a certain range. ? If ciprofloxacin is given to a patient on dialysis, will dialysis increase drug clearance, and if so, what dose is required to replace losses. ? If a patient has an NG tube, can I crush a nifedipine PA tablet Will the patient experience the same clinical response … or must I convert to IV therapy. Simpler questions ? If a patient is taking a herbal medication … will it interfere with other drug therapy? ? If you have 10 different herbal preparations on the shelf – what one is best? ? If the manufacturer of a $300 /IV dose drug says that the drug must be discarded if it is not used immediately, should I discard the medication? ? Can dolasetron and dexamethasone be mixed in the same IV container prior to administration to a patient. All studies have been completed through collaboration in fact for most … the idea/the need was generated by someone else a pharmacist … a physician … someone with a practical question Scientific Papers John Iazzetta 21 Tom Paton 16 Bill Bartle 11 Sandra Tailor 5 Brian Hardy 2 Sharon Yamashita 1 Abstracts 11 14 12 26 9 5 Graduate Students Lee Dupuis Yana Bacjar Ina Sungaila Debra Kwan Carrie Fung Fran Paradiso-Hardy Robert Lepage Information current to December 2001 Examples of studies completed A pharmacist … a physician asks a question, we try to respond 1. 2. 3. 4. 5. 6. Compatibility Study Stability Studies Bioequivalence study Iron Tyramine in food Evaluation of content of Herbals Use of handhelds in Clinical Pharmacy Compatibility Studies. In hospitals, most patients will only have 1 intravenous line running at anyone time. What do you do when a patient has multiple intravenous drugs? Can you give them together ? … or will they ppte in the line? You could given them sequentially … and then flush the line with saline. But this will require excessive nursing time. You could start a second intravenous line …patient? You could test the compatibility. Most studies look at two drugs and present the results in a table Hydromorphone Cefazolin 20 mg/mL in D5W Cefazolin 300 mg/mL I recon vial 2 mg/mL 10 mg/mL 40mg/mL C I C I C I Compatibility of dexamethasone sodium phosphate with hydromorphone hydrochloride or diphenhydramine hydrochloride SCOTT E. WALKER, CARLO DEANGELIS, JOHN IAZZETTA, J. GARY EPPEL Am J Hosp Pharm 48: 2161-6; 1991 When equal volume of dexamethasone (10 mg/mL) and diphenhydramine (50 mg/mL) are mixed a clear but water immiscible liquid is observed. This incompatibility is not visually apparent at lower concentrations. … is this due to compatibility or visual acuity? Equal vol. hydromorphone 50 mg/mL and dexamethasone 2 mg/mL are compatible … but hydromorphone 40 mg/mL and dexamethasone 10 mg/mL are incompatible Are these examples of concentration dependant compatibilities? Develop a method for hydromorphone (H), Dexamethasone (Dx), methylparaben (M), propyl paraben (P), creatinine (C) and degradation products (Dx-1) Then measure concentrations. Dexamethasone & Diphenydramine Equal volumes of D 50 & Dex 10 Equal volumes of D 50 & Dex 4 Region of Incompatibility Region of Compatibility Compatibility Studies Similar graphs have been published for Hydromorphone & Heparin Dexamethasone & Diphenhydramine Dexamethasone & Midazolam Insulin & Labetalol Insulin & Norepinephrine Insulin & dopamine Heparin & Labetolol Heparin & dopamine Stability Studies Manufacturers provide many intravenous drugs as lyophilized powders. … monograph states that following reconstitution that the product should be used immediately (with 24 hours). In most hospital settings, this can contribute to wastage. 24 hours is too little time for a change in dose or a discontinued (unused) IV medication to be re-labeled and given to a second patient. Can the expiry date be extended? Wastage seems to be related to the Shelf-life. You need time to get the IV dose to a second patient. But … can you move a drug along the line? Stability Studies Each dose ~ $30. The top dug on the list in 1986 was cefazolin. With wastage at only 4% monthly expenditures > $16,500. If we used a 24 hour expiry date, wastage could have been ~2640 / month ($31,600 per year). But … can you move a drug along the line? And why would you want to? Systematic evaluation of stability and completed the studies which demonstrated expiry dates could be extended to 7 days… or more. Wastage was eventually reduced to less than 3% Savings in $ 1986 80,000.00 2001 148,000.00 Bioavailability Studies Head of Hematology presents two cases of anemia unresponsive to Enteric Coated Ferrous Sulfate tablets Poor clinical response to enteric-coated iron preparations. RUDINSKAS L, PATON TW, WALKER SE, DOTTEN DA, COWAN DH. Can Med Assoc J. 1989. 141: 565-566. Problem was that patients responded well & quickly to oral liquid formulations of ferrous sulfate. Was it possible that Enteric Coated Ferrous Sulfate Tablets were not being absorbed? … but they had been on the market since the mid 60’s..!! Bioavailability of oral ferrous sulfate preparations in normal volunteers. WALKER SE, PATON TW, COWAN DH, MANUEL MA, AND DRANITSARIS G. Can Med Assoc J. 1989. 141: 543-547. Enteric Coated FeSO4 tablets had bioavailability of less than 25% … a negative bioavailability was estimated for 1 brand DQTC listing Label Accuracy of Herbal Products Your pharmacy stocks several brands of a particular herbal preparation. What brand should you recommend to patients? Or if you only have limited space … what brand should you stock? A January 2000 STAR report evaluated 10 brands of each of ginseng, garlic and feverfew, finding between 0% ~85% of label claim in each. “Its open season on the consumer. When you buy one of these products, it’s an act of faith” Estimating the Concentration of Hypericin(s) in Commercial Products 54 different St John’s wort preparations were purchased 14 US preparations (no tinctures) 4 Walmart (Pittsburgh) 7 Rite Aid Pharmacy in Virginia Beach 3 From 2 other Pharmacies in Virginia Beach 40 Products purchased from Retail Stores in GTA Pharmacies, Health Food Stores, Grocery Stores 12 Tablets 26 Capsules 16 Tinctures Hypericin and pseudohypericin concentrations were determined by chromatography CAPSULE RESULTS 26 Formulations Mean Potency: 56.67% Naturalife Herb Tech Range: 0 % - 108.62% Canadian Potency: 50.26% US Potency: 60.63% p = 0.2611 TABLETS TINCTURES RESULTS 16 Formulations The median concentration g/ml. Mean Potency: of a tincture was 6.49 Mean Concentration: 12 Formulations 53.57% 23.1 g/mL In order to provide a dose similar to the Range: Range: median capsule or tablet, 110 g/mL you 5.46% would - 81.35% have to ingest more than 70 mL0/-dose. 5 mL of Herbalbottles Factors: delivers 550 g Most tinctures come in dropper of 50 mL or less. Label Rec. 1 mL Equivalent to average Tab or cap LIMITATIONS Analysis consisted of only 1 lot Only naphthodianthrones measured Next study evaluated the parthenolide content in Feverfew products, looking at different lots. Most Feverfew products are standardized to 0.2% but some are as high as 0.7% parthenolide 44 different lots of Feverfew products 14 brands of capsules (23 lots), 9 brands tablet/caplet (15 lots) and 6 Tinctures (8 lots) All purchased in Southern Ontario, 1999 Feverfew Products Range of Label Claims Percent of Label Claim Across all brands < 8.4 % to 446% Brands: 14 Caps; 9 Tabs; 6 Tinctures No label claim for 5 Caps and all 6 Tinctures Median ~72% 2/3 are less than 100% 18 brands, 30 different lots with label claims Percent of label claim Capsules 0-> ALBI (Migra Stress) Enzymatic Therapy (Mygracare) Herbal Factors (Feverfew Grand Camomile) Nature’s Way(Feverfew Leaf) Pharma Plus (feverfew)3 Solaray (MigraGard) Solgar (Feverfew Leaf Extract) Swiss (Feverfew 125) Webber Naturals(Feverfew Extract) Tablets Ashbury Biologics (Tanacet 125) Body Basics(Standardized Feverfew Leaf) Flora (Myranon Feverfew Extract) Jamieson (Migraban Feverfew) Life (Nomigraine Matricare) Nature’s Way (MygraFew) Nu-Source Quest (Herbal Migraine Formula) Wellsprings/Quest (Herbal Migraine Formula) 20 40 60 80 100 120 140 160 180 200 300 400 2 2 Feverfew Products Range of Label Claims within a brand Percent of Label Claim 17% 42% 110% Brands: 14 Caps; 9 Tabs; 6 Tinctures 101% 169% 184% No label claim for 5 Caps and all 6 Tinctures 18 brands, 30 different lots with label claims Percent of label claim Capsules 0-> ALBI (Migra Stress) Enzymatic Therapy (Mygracare) Herbal Factors (Feverfew Grand Camomile) Nature’s Way(Feverfew Leaf) Pharma Plus (feverfew)3 Solaray (MigraGard) Solgar (Feverfew Leaf Extract) Swiss (Feverfew 125) Webber Naturals(Feverfew Extract) Tablets Ashbury Biologics (Tanacet 125) Body Basics(Standardized Feverfew Leaf) Flora (Myranon Feverfew Extract) Jamieson (Migraban Feverfew) Life (Nomigraine Matricare) Nature’s Way (MygraFew) Nu-Source Quest (Herbal Migraine Formula) Wellsprings/Quest (Herbal Migraine Formula) 20 40 60 80 100 120 140 160 180 200 300 400 2 2 Range of Label Claims Within a Brand 418 & 446% 58, 65 & 66% Alternative Product Content Result Summary St Johns Wort. 54 brands, single lots Content varies from 0-108% of Label Claim Feverfew 29 brands, 44 lots Content varies from < 8.4% to 446% of label Claim Valerian 19 brands, 30 lots Content varies from 36% to 227% of label Claim Glucosamine 53 brands, 77 lots Content varies from 39% to 134% of label Claim Tyramine Content of Food Why? In normals, ~450 mg of tyramine increases BP 30 mmHg in 50% of subjects In patient on MAO inhibitors, 8 mg increased blood pressure by 30 mmHg. “cheese effect was reported in ~1963 … hypertensive crisis following ingestion of cheese. Severe headache … nausea …some deaths Problem… case reports of patients on MAOI with headache following ingestion of a food… restriction of the food “restricted” food list grows to include 200+ items. Question Experimentation by patients is dangerous Can we produce a list that contains only tryramine containing foods? Tyramine Content of Food Dietary restriction, tyramine and the use of monoamine oxidase inhibitors Shulman KI, Walker SE, MacKenzie S and Knowles S. J clin Psychopharmacol. 1989. 9: 397-402. Analysed 111 foods. Identified several foods (some immature, non-aged cheeses and Chianti Wine) that did not require restriction Hypertensive Episode Associated with phenelzine and Tap Beer A re-analysis of the Role of Pressor Amines in Beer. Tailor, SAN, Shulman KI, Walker SE, Moss J, Gardner D. J Clin Psycopharmacol 1994; 14: 5-14 79 brands of beer, 98 different samples 38 different brands of tap beers, 30 different canned & bottled beer, 11 dealcoholized beers 4 tap beers containing more than 27 mg/L of tyramine Pizza, “combination foods” … sausage …. Handhelds in Pharmacy Organization tools Email, Date/Appointments / Addresses & phone Numbers Medical based programs ePocrates RxTM, Clinical Drug Database ePocrates RxTM, Infectious Disease Guide Patient Tracking Software Pendragon software ePatient 2000 Could you get these devices & programs to help you work more efficiently? Identify patients at risk, gather workload stats, Calculate the cost savings of a clinical recommendation… Other Current Studies Clinical Trials: Evaluation of the Effectiveness of TDM for PI in HIV patients Weekly analysis of PI concentrations in plasma and integration of concentration with HIV virus phenotype to adjust PI dosing. Evaluation of THC to improve appetite in HIV patients Development and analysis of THC, OH & COOH metabolite concentrations in 32 subjects receiving multiple doses of 2%, 4%, & 8% Stability / Compatibility / Analytical Studies: Compatibility & Stability of Pantoprazole Stability of a new formulation of Docetaxel Sources of Funding Pharmaceutical Firms Peer - Review Agencies Ontario Ministry of Heath Ontario HIV Treatment Network Physician Services Incorporated Canadian Institute of Health Research Local Hospital Funding Profit from Pharmaceutical Contract work Collaborators at Sunnybrook John Iazzetta Tom Paton Bill Bartle Sandra Tailor Brian Hardy Sharon Yamashita