The Chemistry of Life REVIEW
advertisement

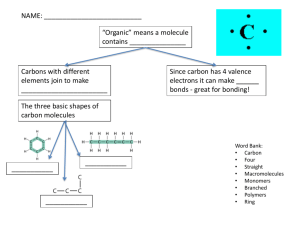
The Chemistry of Life REVIEW Atoms of the same element that differ in the number of neutrons they contain are known as ̶ • isotopes Strong forces bind protons and neutrons together to form the ̶ • nucleus • Weaker Van der Waals forces are (stronger or weaker) than chemical bonds. What kind of bonding does the diagram depict? • Ionic bonding There are ____ electrons being shared between the Carbon atoms. This is referred to as a ____ covalent bond. • Six, triple compound ______ A is a substance formed by the chemical combination of two or more elements in definite proportions. The atomic structure of a water molecule is useful in demonstrating what kinds of bonds? • Covalent bonds gkljfgjsdgkjdafgkjdfs gjkafgfadjkgadkjlgalk fadjladlghfadghfadlkj gkljfgjsdgkjdafgkjdfs gjkafgfadjkgadkjlgalk fadjladlghfadghfadlkj How many neutrons does the diagram of this atom show? • four OH¯ is a(n) ____ as signified by the negative charge. • ion or anion A molecule in which the charges are unevenly distributed is called a(n) polar _______ molecule. jvbjhvhvb jvbjhvhvb What kind of electron is in the last shell or energy level of an atom? • valence hydrogen, oxygen In a water molecule, the ______ end is slightly positive while the ______ end is slightly negative. Because the number of positive ions is equal to the number of negative hydroxide ions neutral produced, water is _______. Water droplets on pine needles are adhesion in good demonstration of _________ that they are different substances. Salt and Water make a solution as Dirt and suspension Water make a _______. As demonstrated by the picture, oil is known to Hydrophobic in be _________ that it repels, or hates, water. Study the two beakers of A solution. Beaker B has (more, less or the same) amount of solute and has (more, less or the same) amount of solvent than the same, beaker A. B more What property of water enables this large tarantula to stay on the surface? • cohesion What does pH measure? (hint: think about what pH is an abbreviation for) the concentration of H+ ions in solution. A(n) ______ is a compound that produces hydroxide ions (OH¯) in solution while a(n) _______ forms H+ ions in solution. base, acid The particles in a (solution or suspension) can be filtered out while the particles in a (solution or suspension) can not. suspension, solution Vinegar is to a(n) (acid or base) as ammonia is to a(n) (acid or base). Acid, base. Acetic acid is the main component in vinegar. The strong acid hydrogen fluoride (HF) can be dissolved in pure water. Will the pH of the solution be greater or less than 7.0 and why? Less than 7.0 because water is already neutral and it will now be a weak acid. Organic chemistry is the study of all compounds that contain bonds between carbon atoms. ______ Structural support provided by what macromolecule includes membranes, hair and nails? proteins Macromolecules are formed by a process known as polymerization __________. What compounds consist of carbon, hydrogen, and oxygen atoms in a 1:2:1 ratio? • Carbohydrates Amino Acids are bonded peptide together by ________ bonds. Amino Acids (aa) aa1 aa2 aa3 aa4 aa5 aa6 What macromolecule does the diagram depict? starch or carbohydrate Galactose, fructose and glucose are all examples of monosaccharides ________ in that they are single sugar molecules. Galactose is a component of milk! What is the polymer of the following monomer? Nucleic Acid fdjnfaskdjfnasjdfabfdljkanfdj What is the metabolic process by which the ‘breaking down’ of complex molecules occurs? Catabolism Cellulose is a polysaccharide plants that found in ______ provides strength. glucose glucose glucose glucose cellulose glucose glucose glucose glucose Lipid A is a _____ fatty acid because it does not have double bonds. fodjndkfngadf A • saturated fodjndkfngadf B What kind of macromolecule is chitin? carbohydrate Which macromolecule ‘stores the most energy’? lipids • Quarternary or 4° Hemoglobin is the iron-containing oxygen-transport metalloprotein in the red blood cells of vertebrates. What kind of protein structure does it demonstrate? Lipids such as olive oil, which contains unsaturated fatty ________ acids, tend to be liquid at room temperature. Carboydrates: Starch is to Polymer as Glucose is to Monomer. ______ Examples of foods containing a high proportion of __________ _________ include cream, cheese, butter, and ghee; suet, tallow, and lard. Saturated fat Did you know??? People make sculptures made of butter.. Check it out! The following monomer is An amino referred to as - acid In the amino acid diagram, the red circle is the _____ group while the blue circle is the ______ group. amino, carboxyl In protein structure, what two levels are missing below in order following the Primary structure? ________?_____ kdjbfsafksadbfkab kdjbfsafksb _______?_____ Secondary and tertiary When the level of glucose in your blood runs low, glycogen is released from your liver. It is referred to as an animal starch. This would make it a polysaccharide __________ in that it is made of many sugar units. Proteins: The bottom secondary structure is an alpha helix while the top structure is Beta _______ pleated _______. sheet a _______ What monomers make up a nucleic acid? gfshssfhgsfhsfhsfhsfhsf nucleotides Amino Acid = Monomer __________ Protein = Polymer Complete the analogy. The elements or compounds that enter into a chemical reaction are known as reactants H₂CO₃ → CO₂ + H₂O Reactants ? Products Lipid molecules are made of ______ ______ and ______. • Fatty acids and glycerol In the following reaction, is energy being absorbed or released? absorbed Endothermic reaction= absorbs energy Exothermic reaction: releases energy What kind of reaction would you classify this as? Exothermic A catalyst changes the rate at which a reaction occurs by increasing it. Therefore, does the catalyst (decrease or increase) the reaction’s activation energy? Decrease A is the _____ while B is the _____. A fdajaklgbf fdajaklgbf B A is the substrate B is the enzyme The substrate attaches to the active enzyme at the ______ site. dkfjbg What three factors can change the productivity, or how well it works, of an enzyme? • pH, temperature, and salinity The following protein has denatured in that it has been ________ unwound or the coils have come undone. bgfxbfgfxhxfghxfgh TIE-BREAKER What enzyme catalyzes the hydrolysis of sucrose to fructose and glucose. Sucrase