Formal Charge
advertisement
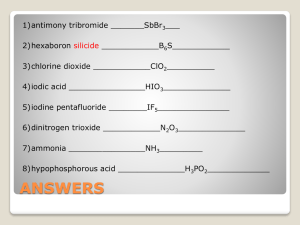
Formal Charge MAKE SURE YOU HAVE THE CORRECT STRUCTURE Formal Charge The Formal Charge of an atom is the charge that an atom in a molecule would have if all atoms had the same electronegativity. The “Big Idea” – you want to draw a structure where the formal charges are equal to zero, or as low as possible if you cannot achieve zero. If you cannot achieve zero, the negative formal charge should be on the most electronegative atom Calculating Formal Charge 1. All of the unshared (nonbonding) electrons are assigned to the atom on which they are found. 2. Half of the bonding electrons are assigned to each atom in the bond. 3. Formal Charge = # of valence e- in the isolated atom minus # of electrons assigned to the atom in the Lewis Structure. Example Draw the Lewis Structure for CO2 O VE 6 AE 6 FC 0 C O 4 or O C O 6 6 4 6 4 6 7 4 5 0 0 -1 0 1 Another Example Sulfate Ion 2- 2- -1 O O -1 O S +2 O -1 O 0 -1 O S -1 0 O -1 O 0 Try H2CO2 O H C O H O Preferred H C O H