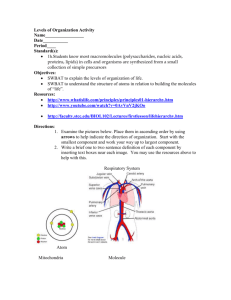
Unit Vision - Real Talk Interactive
advertisement
UNIT VISION - CHEMISTRY Mississippi Delta CRS Standards LEARNING GOALS Unit 6 – MiniUnits: Acids/Bases, Oxidation/Reduction, & Thermodynamics 5a. Analyze and explain acid/base reactions. (DOK 2) Properties of acids and bases, including how they affect indicators and the relative pH of the solution Formation of acidic and basic solutions Definition of pH in terms of the hydronium ion concentration and the hydroxide ion concentration The pH or pOH from the hydrogen ion or hydroxide ion concentrations of solution How a buffer works and examples of buffer solutions 5b. Classify species in aqueous solutions according to the Arrhenius and Bronsted-Lowry definitions, respectively and predict products for aqueous neutralization reactions. (DOK 2) 4a. Explain the thermodynamics associated with physical and chemical concepts related to temperature, entropy, enth energy. (DOK 2) Specific heat as it relates to the conservation of energy Amount of heat absorbed or released in a process, given mass, specific heat, and temperature change Energy (in calories and joules) required to change the state of a sample of a given substance, using its mass vaporization or heat of fusion. Endothermic or exothermic changes 5c. Analyze a reduction/oxidation reaction (REDOX) to assign oxidation numbers (states) to reaction species and identify the and reduced, the oxidizing agent, and reducing agent. (DOK 2) Interpretation of Data None Scientific Investigation None Evaluation of Models, Inferences, and Experimental Results Select a simple hypothesis, prediction, or conclusion that is supported by two or more data or modles Determine whether given information supports or contradicts a simple hypothesis or concl Identify strengths weaknesses in one or more models Identify similarities and differences between models Determine which model(s) is(are) supported or weakened by new information Select a data presentation or a model that supports or contradicts a hypothesis, prediction, Unit 6: Essential Questions 1) How does Chemistry relate to the real world? Revised: December 31, 2011 Unit 6: Big Ides 1) Real world application UNIT VISION - CHEMISTRY Mississippi Delta Day 1 – Bronsted&Arrhenius (90min) Essential Questions 1) Why does skin burn from acid? Key Points Knowledge 1. Bronsted Acids are H+ donors and bases are H+ a 2. Arrhenius: Acids are H+ donors and bases are OH Big Ideas 1) real world application Skills Objectives SWBAT identify Bronsted and Arrhenius acid and bases (DOK2) New CRS: Identify strengths weaknesses in one or more models 1. Identify the acid and base in a Bronsted acid-base re 1) Find a match in the reaction 2) Determine if the molecule in the reactants gaine 3) Draw an arrow of where the H goes in the reacti 4) The acid loses and the base gains the H+ 2. Identify the acid and base in an Arrhenius acid-base 1) Determine if the reaction is Arrhenius (has H2O i 2) The reactant with the -OH is the base 3) The reactant with the H+ is the acid 3. Predict the products of an Arrhenius acid-base react 1) Put H2O in the reactants 2) Cross out the H and the OH in the reactants 3) Combine the remaining ions of the reactants to c Day 2 – Properties (45min) Essential Questions 1) Are household products acidic or basic? Big Ideas 1) real world application Objectives SWBAT name properties of acids and bases (DOK1) New CRS: Identify similarities and differences between models Key Points Knowledge 1. Acids have a sour taste. Bases have a bitter tast 2. Indicators turn a substance a color indicating w acid or a base 3. Cabbage juice is an indicator and turns acids red green 4. The pH of a substance + the pOH of a substance Skills 1. Interpreting Solubility Curves 1) Mark the point on the line that is the grams an of the solution 2) Determine if the point is over, under, or on th solute 3) If it is over = supersaturated; on = saturated; unsaturated Day 3 – Concentration (90min) Revised: December 31, 2011 UNIT VISION - CHEMISTRY Mississippi Delta Essential Questions 1) What’s the difference between rain and acid rain? Big Ideas 1) real world application Objectives SWBAT describe how pH and pOH change with concentration changes (DOK3) Key Points Knowledge 1. Sometimes you have extra H= or OH- which makes and basic. 2. Concentration: the amount of solute in a solvent 3. pH measures the concentration of H= in a water. 4. pOH measures the concentration of OH- in water. 5. If you have a pH of X, then you have a concentrati 6. For every time you increase or decrease the pH by or decrease the concentration of the solution by a New CRS: Determine which model(s) is(are) supported or weakened by new information Day 4 – Titration (90min) Essential Questions 1) How do you neutralize a pool? Big Ideas 1) real world application Objectives SWBAT perform a titration. (DOK2) CRS: none Key Points Knowledge 1. To represent the concentration or molarity of a su around the substance. E.g., the concentration of H represented as [H+]. 2. A solution with equal H= and OH- is neutral. 3. A titration is an experiment where one creates a s equal moles of H+ atoms and OH- atoms. Titration in a Lab A. Put indicator in your solution to see if the solution basic. B. Slowly add a base or acid until the indicator tells it turning green. 4. To read a Buret look at the bottom of the miniscu 5. To determine the amount of a solution added sub reading from the final reading. 6. Because you trying to make the concentrations an to each other use the equation M1V1=M2V2 Skills 1. For any titration problem 1) Find your variables 2) Use the equation M1V1=M2V2 3) Multiply any concentration in the equation by t to make the solution neutral 4) Plug and Chug Day 5 – Oxidation & Reduction (90min) Revised: December 31, 2011 UNIT VISION - CHEMISTRY Mississippi Delta Essential Questions 1) How is fire made? Key Points Knowledge 1. If an element gains an electron then it goes throug Big Ideas 1) real world application 2. 3. 4. 5. Objectives SWBAT write half-reactions. (DOK2) If an element loses and electron then it goes throu OIL – oxidation is loss RIG – reduction is gain To measure electrical current use voltage (V) 6. During chemical reactions energy is always released 7. Endothermic Reactions – energy is used to 8. Exothermic Reactions – energy is released bonds CRS: none Skills 1. Writing electrons in equations Think of half reactions as money (electrons) in an Easte 1) If you gain an electron the money is outside the egg at inside. Egg $1 Egg -1 2) If you lose an electron, the money is in the egg at first outside Egg Egg +1 $1 Day 6 – Voltaic Cells(90min) Essential Questions 1) How does a battery work? Big Ideas 1) real world application Objectives SWBAT solve the final charge of two voltaic cells (DOK2) New CRS: Select a data presentation or a model that supports or contradicts a hypothesis, prediction, or conclusion. Revised: December 31, 2011 Knowledge none Key Points Skills 1. Determine the voltage of two chemical cells Goal: Make sure the number of electrons on each side of balanced. 1) If you need to flip an equation, invert the charge ( goes to negative) 2) If you need to multiply a side, multiply the entire the number. DO NOT MULTIPLY THE VOLTAGE 3) Cancel out the electrons and drop the remaining e final reaction. 4) Add the final voltage together Hint: YOU CANNOT HAVE A NEGATIVE VOLTAGE! UNIT VISION - CHEMISTRY Mississippi Delta Day 7 – Oxidation States (90min) Essential Questions 1) Who is the sea monster GHOG? Key Points Knowledge none Big Ideas 1) real world application Skills Objectives SWBAT determine oxidation state of atoms. (DOK3) 2. Tracking electrons in an equation (oxidation states) 1) If an element is by itself, its charge is zero. 2) If there is a polyatomic ion, the charge will be g 3) +1 G: Group 1 (Na, Li, K, etc.) +1 H: Hydrogen CRS: none -2 O: Oxygen -1 G: Group 7 (F, Cl, Br, etc.) Hint: Make sure the charges of each molecule i to see how an atom changes only look at on Day 8 – Thermodynamics (90min) Essential Questions 1) How can you calculate how much heat you need to melt or vaporize a substance? Big Ideas 1) real world application Objectives SWBAT calculate heat changes in substances (DOK2) CRS: Select a simple hypothesis, prediction, or conclusion that is supported by two or more data presentations or modles Revised: December 31, 2011 Key Points Knowledge 1. 2. 3. 4. The unit for energy is J and the variable is jo The Δ represents change. There is always an initial state and a final st Heat of fusion (ΔHfus) is the amount of heat convert between a solid and a liquid 5. Heat of vaporization (ΔHvap) is the amount o takes to convert between a liquid and a gas 6. To measure how much heat is required to v a substance use q=mΔHfus or q=mΔHvap Skills 1. Predict the outcome of a change at equilibrium 1) Create a mound (extra) or hole (loss) for your 2) If a substance is removed, the reaction will sh removed substance If a substance is added, the reaction will shift away fr substance UNIT VISION - CHEMISTRY Mississippi Delta Day 9 – Specific Heat (90min) Essential Questions 3) Why can some things melt at a lower temperature? Knowledge 1. Calorie: The amount of heat (q) needed to raise 1o degrees Celsius. 2. Big Ideas 3) Overlapping science Objectives SWBAT calculate specific heat (DOK2) CRS: none Key Points The variable to represent specific heat capacity is c. 3. ∆T = Ti - Tf 4. Specific Heat Formula: q=mc∆T Skills 1. Solve word problems involving specific heat 1) Circle variables 2) Create variable list 3) Write the equation 4) Solve for the missing variable 5) Plug and chug Day 10 –Unit Review (90min) Day 11 – Unit Assessment and Lab (90min) Unit Essential Question Sample Answers The last unit is a sampling of extra Chemistry topics, and essential questions are all application based to increase investment/interest in Chemistry beyond Chemistry 1. Complete List of Key Points for Unit 5: Solutions and Gases Knowledge 1. Bronsted Acids are H+ donors and bases are H+ acceptors. 2. Arrhenius: Acids are H+ donors and bases are OH- donors. 3. Acid base neutralization is a double replacement reaction. 4. 5. 6. 7. 8. Acid base neutralizations always create H2O and a salt Acids have a sour taste. Bases have a bitter taste. Indicators turn a substance a color indicating whether it is an acid or a base Cabbage juice is an indicator and turns acids red and bases green The pH of a substance + the pOH of a substance equals 14 Revised: December 31, 2011 UNIT VISION - CHEMISTRY Mississippi Delta Sometimes you have extra H= or OH- which makes a solution acidic and basic. Concentration: the amount of solute in a solvent pH measures the concentration of H= in a water. pOH measures the concentration of OH- in water. If you have a pH of X, then you have a concentration of 1 x 10-x For every time you increase or decrease the pH by 1 you increase or decrease the concentration of the solution by a factor of x10 15. To represent the concentration or molarity of a substance put _____________ around the substance. E.g., the concentration of H+ ions can be represented as [H+]. 16. A solution with equal H= and OH- is neutral. 17. A titration is an experiment where one creates a solution with equal moles of H+ atoms and OH- atoms. Titration in a Lab C. Put indicator in your solution to see if the solution is acidic or basic. D. Slowly add a base or acid until the indicator tells its neutral by turning green. 18. To read a Buret look at the bottom of the miniscus 19. To determine the amount of a solution added subtract the initial reading from the final reading. 20. Because you trying to make the concentrations and volumes equal to each other use the equation M1V1=M2V2 21. If an element gains an electron then it goes through reduction 9. 10. 11. 12. 13. 14. 22. 23. 24. 25. If an element loses and electron then it goes through oxidation OIL – oxidation is loss RIG – reduction is gain To measure electrical current use voltage (V) 26. During chemical reactions energy is always used or released 27. Endothermic Reactions – energy is used to break bonds 28. Exothermic Reactions – energy is released to create new bonds 29. The unit for energy is J and the variable is joules 30. The Δ represents change. 31. There is always an initial state and a final state. 32. Heat of fusion (ΔHfus) is the amount of heat to it takes to convert between a solid and a liquid 33. Heat of vaporization (ΔHvap) is the amount of heat to it takes to convert between a liquid and a gas 34. To measure how much heat is required to vaporize or fuse a substance use q=mΔHfus or q=mΔHvap 35. 36. 37. 38. Calorie: The amount of heat (q) needed to raise a substance by 1o degrees Celsius. The variable to represent specific heat capacity is c. ∆T = Ti - Tf Specific Heat Formula: q=mc∆T Skills 1. Identify the acid and base in a Bronsted acid-base reaction 1) Find a match in the reaction 2) Determine if the molecule in the reactants gained or lost an H Revised: December 31, 2011 UNIT VISION - CHEMISTRY Mississippi Delta 3) Draw an arrow of where the H goes in the reaction 4) The acid loses and the base gains the H+ 2. Identify the acid and base in an Arrhenius acid-base reaction 1) Determine if the reaction is Arrhenius (has H2O in products) 2) The reactant with the -OH is the base 3) The reactant with the H+ is the acid 3. Predict the products of an Arrhenius acid-base reaction 1) Put H2O in the reactants 2) Cross out the H and the OH in the reactants 3) Combine the remaining ions of the reactants to create the salt 4. For any titration problem 1) Find your variables 2) Use the equation M1V1=M2V2 3) Multiply any concentration in the equation by the moles needed to make the solution neutral 4) Plug and Chug 5. Writing electrons in equations Think of half reactions as money (electrons) in an Easter egg (atom). 3) If you gain an electron the money is outside the egg at first, then goes inside. Egg $1 Egg -1 4) If you lose an electron, the money is in the egg at first, the goes outside Egg Egg +1 $1 6. Determine the voltage of two chemical cells Goal: Make sure the number of electrons on each side of the equation is balanced. equation, invert the charge (e.g., positive goes to negative) 5) If you need to flip an 6) If you need to multiply a side, multiply the entire half reaction by the number. DO NOT MULTIPLY THE VOLTAGE 7) Cancel out the electrons and drop the remaining elements in the final reaction. 8) Add the final voltage together Hint: YOU CANNOT HAVE A NEGATIVE VOLTAGE! 7. Tracking electrons in an equation (oxidation states) 1) If an element is by itself, its charge is zero. 2) If there is a polyatomic ion, the charge will be given. 3) +1 G: Group 1 (Na, Li, K, etc.) +1 H: Hydrogen -2 O: Oxygen -1 G: Group 7 (F, Cl, Br, etc.) Hint: Make sure the charges of each molecule is neutral, but to see how an atom changes only look at one atom Revised: December 31, 2011 UNIT VISION - CHEMISTRY Mississippi Delta 8. Solve word problems involving specific heat 1) Circle variables 2) Create variable list 3) Write the equation 4) Solve for the missing variable 5) Plug and chug Revised: December 31, 2011