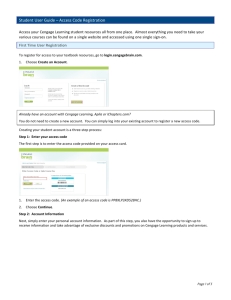
Chapter 19
Electrochemistry
A voltaic cell employs a spontaneous oxidation–
reduction reaction as a source of energy. It separates
the reaction into two half-reactions, physically
separating one half-reaction from the other.
Copyright © Cengage Learning. All rights reserved.
19 | 2
Our first step in studying electrochemical cell is to
balance its oxidation–reduction reaction.
We will use the half-reaction method from Section
4.6 and extend it to acidic or basic solutions.
In this chapter we will focus on electron transfer
rather than proton transfer so the hydronium ion,
H3O+(aq), will be represented by its simpler
notation, H+(aq). Only the notation, not the
chemistry, is different.
Copyright © Cengage Learning. All rights reserved.
19 | 3
We will be studying more complex situations, so our
initial analysis is key.
First, we need to identify what is being oxidized and
what is being reduced.
Then, we determine if the reaction is in acidic or basic
conditions.
Copyright © Cengage Learning. All rights reserved.
19 | 4
The next several topics describe battery cells or
voltaic cells (galvanic cells).
An electrochemical cell is a system consisting
of electrodes that dip into an electrolyte and in
which a chemical reaction either uses or
generates an electric current.
Copyright © Cengage Learning. All rights reserved.
19 | 5
A voltaic or galvanic cell is an electrochemical
cell in which a spontaneous reaction generates
an electric current.
An electrolytic cell is an electrochemical cell in
which an electric current drives an otherwise
nonspontaneous reaction.
Copyright © Cengage Learning. All rights reserved.
19 | 6
Physically, a voltaic cell consists of two half-cells
that are electrically connected.
Each half-cell is the portion of the electrochemical
cell in which a half-reaction takes place.
The electrical connections allow the flow of
electrons from one electrode to the other.
The cells must also have an internal cell
connection, such as a salt bridge, to allow the flow
of ions.
Copyright © Cengage Learning. All rights reserved.
19 | 7
The Cu2+ ions gain
two electrons,
forming solid copper.
The zinc metal atom
loses two electrons,
forming Zn2+ ions.
The electrons flow through the external circuit from
the zinc electrode to the copper electrode.
x
Ions flow through the salt bridge to
maintain charge balance.
Copyright © Cengage Learning. All rights reserved.
19 | 8
A salt bridge is a tube of electrolyte in a gel that is
connected to the two half-cells of the voltaic cell. It
allows the flow of ions but prevents the mixing of
the different solutions that would allow direct
reactions of the cell reactants.
In the salt bridge, cations move toward the
cathode and anions move toward the anode.
Copyright © Cengage Learning. All rights reserved.
19 | 9
The electrode at which oxidation takes place is
called the anode.
The electrode at which reduction takes place is
called the cathode.
Electrons flow through the external circuit from
the anode to the cathode.
Copyright © Cengage Learning. All rights reserved.
19 | 10
Copyright © Cengage Learning. All rights reserved.
19 | 11
This figure below illustrates the same reaction,
replacing the light bulb with a voltmeter. The solution
on the right is now blue from the Cu2+ ion formed.
Copyright © Cengage Learning. All rights reserved.
19 | 12
You construct one half-cell of a voltaic
cell by inserting a copper metal strip
into a solution of copper(II) sulfate. You
construct another half-cell by inserting
an aluminum metal strip in a solution of
aluminum nitrate. You now connect the halfcells by a salt bridge. When connected to an
external circuit, the aluminum is oxidized.
Sketch the resulting voltaic cell. Label the
anode and the cathode, showing the
corresponding half-reactions. Indicate the
direction of electron flow in the external circuit.
?
Copyright © Cengage Learning. All rights reserved.
19 | 13
e-
cathode
Cu
e-
CA2+
NO3-
anode
Al
Cu2+
Al3+
SO42-
NO3-
Cu2+ + 2e- Cu
Copyright © Cengage Learning. All rights reserved.
Al Al3+ + 3e19 | 14
Voltaic cell notation is a shorthand method of
describing a voltaic cell.
The oxidation half-cell, the anode, is written on the
left.
The reduction half-cell, the cathode, is written on the
right.
Copyright © Cengage Learning. All rights reserved.
19 | 15
The cell terminal is written on the outside: left for the
anode and right for the cathode.
Phase boundaries are shown with a single vertical
bar, |.
For example, at the anode, the oxidation of Cu(s) to
Cu2+(aq) is shown as Cu(s) | Cu2+(aq).
At the cathode, the reduction of Zn2+(aq) to Zn(s) is
shown as Zn2+(aq) | Zn(s).
Copyright © Cengage Learning. All rights reserved.
19 | 16
Between the anode and cathode the salt bridge is
represented by two vertical bars, ||.
The complete notation for the reaction is
Cu(s) | Cu2+(aq) || Zn2+(aq) | Zn(s)
Copyright © Cengage Learning. All rights reserved.
19 | 17
When the half-reaction involves a gas, the electrode
is an inert material such as platinum, Pt. It is
included as a third substance in the half-cell.
For example, the half-reaction of Cl2 being reduced
to Cl- is written as follows:
Cl2(g) | Cl-(aq) | Pt
Because this is a reduction, the electrode appears
on the far right.
For the oxidation of H2(g) to H+(aq), the notation is
Pt | H2(g) | H+(aq)
In this case, the electrode appears on the far left.
Copyright © Cengage Learning. All rights reserved.
19 | 18
To fully specify a voltaic cell, it is necessary to give
the concentrations of solutions or ions and the
pressures of gases. In the cell notation, these
values are written within parentheses for each
species.
For example, the oxidation of Cu(s) to Cu2+(aq) at
the anode and the reduction of F2(g) to F-(aq) at
the cathode is written as follows:
Cu(s) | Cu2+(1.0 M) || F2(1.0 atm) | F-(1.0 M) | Pt
Copyright © Cengage Learning. All rights reserved.
19 | 19
?
The cell notation for a voltaic cell is
Al(s) | Al3+(aq) || Cu2+(aq) | Cu(s)
Write the cell reaction.
Our strategy is to write and balance each halfreaction, and then to combine the half-reactions to
give the overall cell reaction.
The skeleton oxidation reaction is Al(s) Al3+(aq).
The skeleton reduction reaction is Cu2+(aq) Cu(s).
Copyright © Cengage Learning. All rights reserved.
19 | 20
Balanced oxidation half-reaction
Al(s) Al3+(aq) + 3eBalanced reduction half-reaction
Cu2+(aq) + 2e- Cu(s)
The common multiple of the electrons is 6. Combine
the half-reactions by multiplying the oxidation halfreaction by 2 and the reduction half-reaction by 3.
Copyright © Cengage Learning. All rights reserved.
19 | 21
2Al(s) 2Al3+(aq) + 6e3Cu2+(aq) + 6e- 3Cu(s)
2Al(s) + 3Cu2+(aq) 2Al3+(aq) + 3Cu(s)
Copyright © Cengage Learning. All rights reserved.
19 | 22
Now we shift our focus to the movement of the
electrons in the oxidation–reduction reaction.
To better understand this movement, we can
compare the flow of electrons to the flow of water.
Copyright © Cengage Learning. All rights reserved.
19 | 23
Just as work is required to pump water from
one point to another, so work is required to
move electrons.
Water flows from areas of high pressure to
areas of low pressure. Similarly, electrons flow
from high electric potential to low electric
potential. Electric potential can be thought of as
electric pressure.
Copyright © Cengage Learning. All rights reserved.
19 | 24
Potential difference is the difference in electric
potential (electrical pressure) between two points.
Potential difference is measured in the SI unit volt
(V).
Electrical work = charge x potential difference
The SI units for this are
J=C×V
J
V
C
Copyright © Cengage Learning. All rights reserved.
19 | 25
The magnitude of charge on one mole of electrons is
given by the Faraday constant, F. It equals 9.6485 ×
103 C per mole of electrons.
1 F = 9.6485 × 103 C
Substituting this into the equation for work:
w = –F × potential difference
The term is negative because the cell is doing work
in creating the current (that is, electron flow).
Copyright © Cengage Learning. All rights reserved.
19 | 26
Normally the potential across the voltaic cell
electrodes is less than the maximum possible.
One reason for this discrepancy is that it takes
energy (work) to drive the current through the cell:
The greater the current, the less the voltage. The cell
has its maximum voltage only when no current flows.
Copyright © Cengage Learning. All rights reserved.
19 | 27
The situation for electrons is analogous to that seen
with water. The difference between water pressure in
the tap and the pressure of the outside atmosphere
is at its maximum when the tap is off; once the tap is
on, the pressure difference decreases.
Copyright © Cengage Learning. All rights reserved.
19 | 28
The maximum potential difference between the
electrodes of a voltaic cell is called the cell potential
or electromotive force (emf) of the cell, or Ecell.
Ecell can be measured with a digital voltmeter. The
anode of a voltaic cell has negative polarity; the
cathode has positive polarity.
Copyright © Cengage Learning. All rights reserved.
19 | 29
The maximum work is given by the following
equation:
Wmax = –nFEcell
Copyright © Cengage Learning. All rights reserved.
19 | 30
?
The emf of a particular cell is 0.500 V.
The cell reaction is
2Al(s) + 3Cu2+(aq) 2Al3+(aq) + 3Cu(s)
Calculate the maximum electrical work
of this cell obtained from 1.00 g of
aluminum.
Copyright © Cengage Learning. All rights reserved.
19 | 31
To determine the value of n (that is, the number of
moles of electrons involved in either half-cell
reaction), we need to examine the half-reactions.
2Al(s) 2Al3+(aq) + 6e3Cu2+(aq) + 6e- 3Cu(s)
n=6
F = 96,485 C/mol
Ecell = 0.500 V = 0.500 J/C
wmax = –nFEcell
wmax = –(6 mol)(96,485 C/mol)(0.500 J/C)
wmax = 2.89 × 105 J
Copyright © Cengage Learning. All rights reserved.
19 | 32
This is the energy corresponding to the balanced
reaction, so we need to convert to one gram of
aluminum.
w max
2.89 x 105 J 1mol Al
5.36 103 J
2 mol Al
26.98 g Al
wmax = 5.36 kJ
Copyright © Cengage Learning. All rights reserved.
19 | 33
A cell potential is a measure of the driving force of
the cell reaction. It is composed of the oxidation
potential for the oxidation half-reaction at the anode
and the reduction potential, for the reduction halfreaction at the cathode.
Ecell = oxidation potential + reduction potential
The oxidation potential for a half-reaction =
–(reduction potential for the reverse half-reaction)
Copyright © Cengage Learning. All rights reserved.
19 | 34
Because the oxidation and reduction potentials are
opposites, we need to calculate only one or the
other. By convention, reduction potentials are
calculated and are called electrode potentials, E
(with no subscript).
Electrode potentials are a measure of the oxidizing
ability of the reactant. Table 19.1 shows the
increasing strength of the oxidizing agents.
Copyright © Cengage Learning. All rights reserved.
19 | 35
Copyright © Cengage Learning. All rights reserved.
19 | 36
Copyright © Cengage Learning. All rights reserved.
19 | 37
Copyright © Cengage Learning. All rights reserved.
19 | 38
Ecell = Eoxidation + Ereduction
Because the electrode potentials are for reduction:
Eoxidation = –Eanode
We can rewrite the equation for the cell:
Ecell = - Eanode + Ecathode
Ecell = Ecathode – Eanode
Copyright © Cengage Learning. All rights reserved.
19 | 39
Let’s find Ecell for the following cell:
Al(s) | Al3+(aq) || Cu2+(aq) | Cu(s)
At the cathode, Cu2+(aq) is reduced to Cu(aq).
E = 0.34 V
At the anode, Al is oxidized to Al3+.
E = –[electrode potential for Al3+(aq)]
E = –(–1.66 V) = 1.66 V
Ecell = 0.34 V + 1.66 V
Ecell = 2.00 V
Copyright © Cengage Learning. All rights reserved.
19 | 40
The standard electrode potential, E°, is the
electrode potential when the concentrations of
solutes are 1 M, the gas pressures are 1 atm, and
the temperature has a specified value (usually
25°C). The superscript degree sign (°) signifies
standard-state conditions.
Copyright © Cengage Learning. All rights reserved.
19 | 41
A fuel cell is simply a voltaic cell that uses a
continuous supply of electrode materials to
provide a continuous supply of electrical
energy. A fuel cell employed by NASA on
spacecraft uses hydrogen and oxygen under basic
conditions to produce electricity. The water produced in
this way can be used for drinking. The net reaction is
?
2H2(g) + O2(g) 2H2O(g)
Calculate the standard emf of the oxygen–hydrogen
fuel cell.
2H2O(l) + 2e- H2(g) + 2OH-(aq) E° = –0.83 V
O2(g) + 2H2O(l) + 4e- 4OH-(aq) E° = 0.40 V
Copyright © Cengage Learning. All rights reserved.
19 | 42
Anode reaction:
2H2O(l) + 2e- H2(g) + 2OH-(aq) E°red = –0.83 V
Cathode reaction:
O2(g) + 2H2O(l) + 4e- 4OH-(aq) E°red = 0.40 V
Overall reaction:
Ecell = Ecathode – Eanode
Ecell = 0.40 V – (–0.83 V)
Ecell = 1.23 V
Copyright © Cengage Learning. All rights reserved.
19 | 43
Comparing Oxidizing Strengths
The oxidizing agent is itself reduced and is the
species on the left of the reduction half-reaction.
Consequently, the strongest oxidizing agent is the
product of the half-reaction with the largest (most
positive) E° value.
Copyright © Cengage Learning. All rights reserved.
19 | 44
Comparing Reducing Strengths
The reducing agent is itself oxidized and is the
species on the right of the reduction half-reaction.
Consequently, the strongest reducing agent is the
reactant in the half-reaction with the smallest (most
negative) E° value.
Copyright © Cengage Learning. All rights reserved.
19 | 45
?
Which is the stronger reducing agent
under standard conditions: Sn2+ (to Sn4+)
or Fe (to Fe2+)?
Which is the stronger oxidizing agent
under standard conditions: Cl2 or MnO4-?
The stronger reducing agent will be oxidized and has
the more negative electrode potential.
The standard (reduction) potentials are
Sn2+ to Sn4+
E = 0.15 V
Fe to Fe2+
E = –0.41 V
The stronger reducing agent is Fe (to Fe2+).
Copyright © Cengage Learning. All rights reserved.
19 | 46
The stronger oxidizing agent will be reduced.
The standard (reduction) potentials are
Cl2 to ClE = 1.36 V
MnO4- to Mn2+ E = 1.49 V
The stronger oxidizing agent is MnO4-.
Copyright © Cengage Learning. All rights reserved.
19 | 47
Predicting the Direction of Reaction
You can predict the direction of reaction by
comparing the relative oxidizing (or reducing)
strengths.
The stronger oxidizing agent will be reduced. (The
stronger reducing agent will be oxidized.)
Copyright © Cengage Learning. All rights reserved.
19 | 48
?
Will dichromate ion oxidize
manganese(II) ion to permanganate ion
in acid solution under standard
conditions?
Standard potentials
Cr2O72- to Cr3+
MnO4- to Mn2+
E° = 1.33 V
E° = 1.49 V
MnO4- has a larger reduction potential; it will oxidize
Cr3+ to Cr2O72-.
Cr2O72- will not oxidize Mn2+ to MnO4-.
Copyright © Cengage Learning. All rights reserved.
19 | 49
?
Let us define the reduction of I2 to Iions, I2(s) + 2e- 2I-(aq), as the
standard reduction reaction with E° =
0.00 V. We then construct a new
standard reduction table based on this
definition.
a. What would be the new standard reduction
potential of H+?
Copyright © Cengage Learning. All rights reserved.
19 | 50
b. Would using a new standard reduction table
change the measured value of freshly prepared
voltaic cell made from Cu and Zn? (Assume you
have the appropriate solutions and equipment to
construct the cell.)
c. Would the calculated voltage for the cell in part b
be different if your were using the values given in
Table 19.1? Do the calculations to justify your
answer.
Copyright © Cengage Learning. All rights reserved.
19 | 51
a. When the H+(aq) E° = 0.000 V, the I2 E° = 0.54 V.
So, if the new I2 potential is 0.000 V, then the H+
potential would be –0.54 V.
b. The measured voltaic cell voltage will be
unchanged.
c. No. The calculated voltage is the same using
either standard because it is a difference is
potential.
Copyright © Cengage Learning. All rights reserved.
19 | 52
The free-energy change, DG, for a reaction equals
the maximum useful work of the reaction.
DG° = wmax = –nFE°
Copyright © Cengage Learning. All rights reserved.
19 | 53
?
Calculate the standard free-energy
change for the net reaction used in the
hydrogen–oxygen fuel cell:
2H2(g) + O2(g) 2H2O(l)
The cell potential is 1.23 V.
How does this compare with DGf° of
H2O(l)?
Copyright © Cengage Learning. All rights reserved.
19 | 54
First we determine the number of electrons involved
in the reaction. Four H are oxidized to H+, and two O
are reduced to O2-. Thus four electrons are involved.
n=4
E° = 1.23 V
DG° = –nFE°
DG = –(4 mol)(96,485 C/mol)(1.23 J/C)
DG° = –4.75 × 105 J
DG° = –475 kJ
DGf° = –285.8 kJ/mol; –550 kJ for 2 mol
Copyright © Cengage Learning. All rights reserved.
19 | 55
?
A voltaic cell consists of one half-cell with Fe
dipping into an aqueous solution of 1.0 M
FeCl2 and the other half-cell with Ag dipping
into an aqueous solution of 1.0 M AgNO3.
Obtain the standard free-energy change for the cell
reaction using the standard free-energy change for
the reaction (found using standard free energies of
formation). The standard free energies of formation
of the ions are Ag+(aq), 77 kJ/mol, and Fe2+(aq), –85
kJ/mol.
What is the cell potential?
Copyright © Cengage Learning. All rights reserved.
19 | 56
We first determine the reaction by using reduction
potentials.
Fe2+(aq), Fe(s) E° = –0.41 V
Ag+(aq), Ag(s)
E° = 0.80 V
Fe is the anode (oxidation).
Ag is the cathode (reduction).
E° = 0.80 – (–0.41) = 1.21 V
The half-reactions are
Fe(s) Fe2+(aq) + 2e2Ag+(aq) + 2e- 2Ag(s)
The overall reaction is
Fe(s) + 2Ag+(aq) Fe2+(aq) + 2Ag(s)
Copyright © Cengage Learning. All rights reserved.
19 | 57
Fe(s) + 2Ag+(aq) Fe2+(aq) + 2Ag(s)
DG° = 1 mol(–85 kJ/mol) – 2 mol(77 kJ/mol)
DG° = –85 kJ – 154 kJ
DG° = –239 kJ
Copyright © Cengage Learning. All rights reserved.
19 | 58
Now we can use this value to find E°.
ΔG
E
nF
J
239,000
mol
E
J
(2 mol) 96,485
V
E 1.24 V
This answer compares favorably to the 1.21 V
determined using standard potentials.
Copyright © Cengage Learning. All rights reserved.
19 | 59
Equilibrium Constants from Cell Potentials
nFE° = RT ln K
Rearranging:
RT
E
ln K
nF
We could now convert from ln to log:
o
cell
2.303 RT
E
log K
nF
0.0592
o
Ecell
log K
n
o
cell
At 25°C:
Copyright © Cengage Learning. All rights reserved.
19 | 60
?
Calculate the equilibrium constant K at
25°C for the following reaction for the
standard cell potential:
Pb2+(aq) + Fe(s) Pb(s) + Fe2+(aq)
First, we find the cell potential.
E°cathode = –0.13 V
E°anode = –0.41 V
(Pb2+)
(Fe2+)
E°cell = E°cathode – E°anode
E°cell = –0.13 V – (–0.41 V)
E°cell = +0.28 V
Now we can find K.
Copyright © Cengage Learning. All rights reserved.
19 | 61
o
cell
nFE
ln K
RT
C
J
2 mol 96,485
0.28
mol
C
ln K
J
8.31
298 K
mol K
ln K = 21.82
K = 3.0 × 109
Copyright © Cengage Learning. All rights reserved.
19 | 62
Summary of
Relationship
Among Variables
Copyright © Cengage Learning. All rights reserved.
19 | 63
Dependence of Cell Potential on Concentration:
Nernst Equation
DG = DG° + RT ln Q
Q is the thermodynamic equilibrium constant.
This equation allows us to relate E to E°.
Ecell E
Copyright © Cengage Learning. All rights reserved.
o
cell
RT
–
ln Q
nF
19 | 64
At 25°C, this reduces to
Ecell E
Copyright © Cengage Learning. All rights reserved.
o
cell
0.02568
–
ln Q
n
19 | 65
Determination of pH
Cell measurements can allow us to determine the
pH of a solution by determining [H+]. This is the
basis of the pH meter.
Copyright © Cengage Learning. All rights reserved.
19 | 66
On the left a small, commercial
electrode is pictured.
On the right is a sketch showing the
construction of a glass electrode for
measuring hydrogen concentrations.
Copyright © Cengage Learning. All rights reserved.
19 | 67
Consider a voltaic cell,
Fe(s) | Fe2+(aq) || Cu2+(aq) | Cu(s),
being run under standard conditions.
a. Is DG° positive or negative for this process?
?
b. Change the concentrations from their
standard values in such a way that Ecell is
reduced. Write your answer using the
shorthand notation.
Copyright © Cengage Learning. All rights reserved.
19 | 68
a.
DG° = –nFE°
E° = 0.36 – (–0.41) = 0.75 V
Since E° is positive, DG° is negative.
b.
Overall reaction:
Fe(s) + Cu2+(aq) Fe2+(aq) + Cu(s)
To reduce Ecell, Q must be > 1.
Fe
Q
Cu
2
2
So, [Fe2+] must be > [Cu2+].
Larger
Smaller
Fe(s) | Fe2+(aq) (1.1 M) || Cu2+(aq) (0.50 M) | Cu(s)
Copyright © Cengage Learning. All rights reserved.
19 | 69
A pH meter is constructed using
hydrogen gas bubbling over an inert
platinum electrode (the hydrogen
electrode) at a pressure of 1.2 atm. The
other electrode is aluminum metal immersed in
a 0.20 M Al3+ solution. What is the cell potential
when the hydrogen electrode is immersed in a
sample of acid rain with a pH of 4.0 at 25°C?
If the electrode is placed in a sample of
shampoo solution and the cell potential is 1.17
V, what is the pH of the shampoo solution? The
reaction is
2Al(s) + 6H+(aq) 2Al3+(aq) + 3H2(g)
?
Copyright © Cengage Learning. All rights reserved.
19 | 70
E E
o
Q
H
3
H2
3 2
P Al
6
o
cell
RT
ln Q
nF
1.2 atm 0.20 M
3
10
-4
2
M
6
= 6.91 × 1022
ln Q = 52.6
0.02568
52.6
E 1.66 –
6
E 1.66 – 0.23
E = 1.43 V
Copyright © Cengage Learning. All rights reserved.
19 | 71
E E
o
o
cell
RT
ln Q
nF
RT
o
ln Q Ecell
Eo
nF
RT
ln Q 1.66 V 1.17 V = 0.49 V
nF
nF
ln Q 0.49 V
RT
C
6 96,485
J
mol
ln Q 0.49
J
C
8.3145
298 K
mol K
Copyright © Cengage Learning. All rights reserved.
19 | 72
ln Q = 114.5
Q = 5.26 × 1049
Q
H
6
3
H2
3 2
P Al
Q
H
3
H2
3 2
P Al
6
1.2 atm 0.20 M
3
2
5.26 10
49
[H+] 6 = 1.31 × 10-51
[H+] = 3.31 × 10-9 M
pH = 8.48
Copyright © Cengage Learning. All rights reserved.
19 | 73
Commercial Voltaic Cells
We next look at several commercial voltaic cells.
Zinc–Carbon Dry Cell: Leclanché
Anode:
Zn(s) Zn2+(aq) + 2eCathode: 2NH4+(aq) + 2MnO2(s) + 2e Mn2O3(s) + H2O(l) + 2NH3(aq)
The initial voltage is about 1.5 V, but decreases and
deteriorates rapidly in cold weather.
Copyright © Cengage Learning. All rights reserved.
19 | 74
Copyright © Cengage Learning. All rights reserved.
19 | 75
Zinc–Carbon Dry Cell: Alkaline
Anode:
Zn(s) + 2OH-(aq) Zn(OH)2(s) + 2eCathode: 2MnO2(s) + H2O(l) + 2e Mn2O3(s) + 2OH- (aq)
This cell performs better under current drain and in
cold weather. It isn’t truly “dry” but rather uses an
aqueous paste.
Copyright © Cengage Learning. All rights reserved.
19 | 76
Copyright © Cengage Learning. All rights reserved.
19 | 77
Lithium–Iodine Battery
In this solid-state battery,
the electrodes are
separated by a thin
crystalline layer of
lithium iodide. Diffusion
of the Li+ ion carries the
current. The cell has
high resistance and,
therefore, low voltage. It
is very reliable and is
used for pacemakers.
Copyright © Cengage Learning. All rights reserved.
19 | 78
Lead Storage Cell
The electrodes are lead alloy grids: one is packed
with a spongy lead to form the anode, and the other
is packed with lead dioxide to form the cathode. Both
electrodes are in an aqueous solution of H2SO4.
Anode:
Cathode:
Pb(s) + HSO4-(aq)
PbSO4(s) + H+(aq) + 2ePbO2(s) + 3H+(aq) + HSO4-(aq) + 2e PbSO4(s) + 2H2O(l)
Unlike dry cells, after discharge, lead storage cells
can be recharged.
Copyright © Cengage Learning. All rights reserved.
19 | 79
Each of the six cells in the lead storage cell
generates 2 V, yielding 12 V. During discharge,
white PbSO4(s) coats each electrode.
To recharge the cell, an external current is used,
reversing the previous reactions. Some water
decomposes into hydrogen and oxygen gas, so
more water may need to be added.
Newer batteries use electrodes with calcium in the
lead, which resists decomposition by water. These
versions are maintenance free.
Copyright © Cengage Learning. All rights reserved.
19 | 80
Copyright © Cengage Learning. All rights reserved.
19 | 81
Nickel–Cadmium Cell
Anode:
Cd(s) + 2OH-(aq) Cd(OH)2(s) + 2eCathode: NiOOH(s) + H2O(l) + e Ni(OH)2(s) + OH-(aq)
These cells are used in calculators,
portable power tools, shavers, and
toothbrushes. During recharge, the
reactions are reversed, which can
be done many times.
When cadmium is replaced with a metal hydride
(MH), nickel metal hydride and lithium hydride cells
result. They are less toxic.
Copyright © Cengage Learning. All rights reserved.
19 | 82
Fuel Cell
Fuel cells require a continuous supply of reactants
(fuel).
Anode:
Cathode:
H2(g) 2H+(aq) + 2eO2(g) + 4H+(aq) + 4e- 2H2O(l)
Fuel cells were originally used in space
applications, but are now being explored for more
uses.
Copyright © Cengage Learning. All rights reserved.
19 | 83
Copyright © Cengage Learning. All rights reserved.
19 | 84
Corrosion Control: Cathodic Protection
Voltaic cells can be used to control corrosion of
underground pipelines and tanks. Rusting occurs
when water comes in contact with iron. The edge
of the water drop, when exposed to air, becomes
one pole of a voltaic cell where oxygen is reduced
to hydroxide.
Anode:
Cathode:
Fe(s) Fe2+(aq) + 2eO2(g) + 2H2O(l) + 4e- 4OH-(aq)
Copyright © Cengage Learning. All rights reserved.
19 | 85
Copyright © Cengage Learning. All rights reserved.
19 | 86
When the buried metal is connected to a more
active metal such as magnesium, the magnesium
becomes the anode and the iron becomes the
cathode. The iron is, therefore, protected from
oxidation.
This phenomenon is called cathodic protection.
Copyright © Cengage Learning. All rights reserved.
19 | 87
Copyright © Cengage Learning. All rights reserved.
19 | 88
Cathodic protection is illustrated below. The nails
are in a gel containing phenolphthalein indicator
and potassium ferricyanide.
Iron corrosion yields Fe2+, which reacts with
ferricyanide ion to give a dark blue precipitate.
Where OH- forms, phenolphthalein appears pink.
Unprotected
Copyright © Cengage Learning. All rights reserved.
Protected
19 | 89
?
Keeping in mind that seawater contains
a number of ions, explain why seawater
corrodes iron much faster than
freshwater.
Many of the ions in seawater have very high
reduction potentials—higher than the reduction
potential of Fe(s). This means spontaneous
electrochemical reactions will occur with Fe(s),
causing the iron to form ions and go into solution. At
the same time, the ions in the sea will be reduced
and plate out on the surface of the iron.
Copyright © Cengage Learning. All rights reserved.
19 | 90
Electrolytic Cell
An electrolytic cell is an electrochemical cell in
which an electric current drives an otherwise
nonspontaneous reaction.
The process of producing a chemical change in an
electrolytic cell is called electrolysis. Many
important substances are produced commercially
by electrolysis—for example, aluminum and
chlorine.
Copyright © Cengage Learning. All rights reserved.
19 | 91
Downs Cell
A Downs cell is an electrolytic cell used to obtain
sodium metal by electrolysis of sodium chloride.
The products must be kept separated or they
would react.
Anode:
Cathode:
Cl-(l) ½Cl2(g) + eNa+(l) + e- Na(l)
Copyright © Cengage Learning. All rights reserved.
19 | 92
Copyright © Cengage Learning. All rights reserved.
19 | 93
Copyright © Cengage Learning. All rights reserved.
19 | 94
Aqueous Electrolysis
For a molten salt, the possible reactions are limited
to those involving the ions from the salt.
In aqueous situations, however, the possible
reactions of water must also be included.
2H2O(l) + 2e- H2(g) + 2OH-(aq)
2H2O(l) O2(g) + 4H+(aq) + 4e-
Copyright © Cengage Learning. All rights reserved.
19 | 95
Unlike voltaic cell reactions, which maximize the
cell potential, the reaction requiring the smallest
voltage will be the one that occurs. To determine
the reaction, it is necessary to consider all possible
half-reactions that might occur.
First, examine the possible oxidation reactions.
The one with the least negative E° value will occur.
Next examine the possible reduction reactions.
The one with the more positive E° value will occur.
Copyright © Cengage Learning. All rights reserved.
19 | 96
Electrolysis of Sulfuric Acid Solutions
Possible oxidation reactions:
2SO42-(aq) S2O82-(aq) + 2eE°red = 2.01 V
2H2O(l) O2(g) + 4H+(aq) + 4e- E°red = 1.23 V
Smaller reduction potential
Possible reduction reactions:
H+(aq) + e- ½ H2(g)
E°red = 0.00 V
2H2O(l) + 2e- H2(g) + 2OH-(aq) E°red = –0.83 V
Larger (more positive) reduction potential
Copyright © Cengage Learning. All rights reserved.
19 | 97
2H2O(l) O2(g) + 4H+(aq) + 4e4H+(aq) + 4e- 2H2(g)
4H+(aq) + 2H2O(l) 2H2(g) + O2(g)
Ecell°= E°cathode – E°anode
Ecell°= 0.00 – 1.23
Ecell°= –1.23 V
Copyright © Cengage Learning. All rights reserved.
19 | 98
Electrolysis of Sodium Chloride Solution
Smaller
reduction potential
Possible oxidation reactions:
2Cl-(aq) Cl2(g) + 2eE°red = 1.36 V
2H2O(l) O2(g) + 4H+(aq) + 4e- E°red = 1.23 V
Possible reduction reactions:
Na+(aq) + e- Na(s)
H+(aq) + e- ½H2(g)
E°red = –2.71 V
E°red = 0.00 V
Larger (more positive) value
Copyright © Cengage Learning. All rights reserved.
19 | 99
Cl2(g) + 2e- 2Cl-(aq)
2H+(aq) + 2e- H2(g)
2H+(aq) + Cl2 (g) H2(g) + 2Cl-(aq)
Ecell°= E°cathode – E°anode
Ecell° = 0.00 – 1.36
Ecell° = –1.36 V
Copyright © Cengage Learning. All rights reserved.
19 | 100
?
Describe what you expect to happen at
the electrodes when an aqueous
solution of sodium iodide is
electrolyzed.
Copyright © Cengage Learning. All rights reserved.
19 | 101
Electrolysis of Sodium Iodide Solution
Smaller
reduction potential
Possible oxidation reactions:
2I-(aq) I2(g) + 2e2H2O(l) O2(g) + 4H+(aq) + 4e-
E°red = 0.54 V
E°red = 1.23 V
Possible reduction reactions:
Na+(aq) + e- Na(s)
E°red = –2.71 V
H+(aq) + e- ½H2(g)
E°red = 0.00 V
Larger (more positive) value
Copyright © Cengage Learning. All rights reserved.
19 | 102
I2(g) + 2e- 2I-(aq)
2H+(aq) + 2e- H2(g)
2H+(aq) + I2 (g) H2(g) + 2I-(aq)
Ecell°= E°cathode – E°anode
Ecell°= 0.00 – 0.54
Ecell°= –0.54 V
Copyright © Cengage Learning. All rights reserved.
19 | 103
The electrolysis of sodium chloride is the basis of
the chlor-alkali industry, which produces chlorine
and sodium hydroxide.
Copyright © Cengage Learning. All rights reserved.
19 | 104
The chlor-alkali mercury cell uses mercury metal
as the cathode. Sodium is reduced, rather than
water, forming a sodium mercury amalgam.
Copyright © Cengage Learning. All rights reserved.
19 | 105
Metals can also be protected from corrosion by
plating them with other metals.
For example, zinc can be plated on steel by
electrogalvanizing to prevent rusting.
Copyright © Cengage Learning. All rights reserved.
19 | 106
Metals can also be purified by electrolysis. Here
we see copper being purified.
Copyright © Cengage Learning. All rights reserved.
19 | 107
Stoichiometry of Electrolysis
In the 1830s, Michael Faraday showed that the
total charge that flows in a circuit is related to the
amount of substance released at the electrodes.
One faraday of charge is the charge on one mole
of electrons and is equal to 96,485 coulombs.
Copyright © Cengage Learning. All rights reserved.
19 | 108
For quantitative considerations, we also need to
know the magnitude of the current and the time it
has flowed.
Electric charge = current × time
Coulombs = amperes × seconds
The ampere, A, is the base unit of current.
The coulomb, C, is equal to an ampere-second.
Copyright © Cengage Learning. All rights reserved.
19 | 109
?
What electric charge is required to
plate a piece of automobile molding
with 1.00 g of chromium metal using a
chromium(III) ion solution?
If the electrolysis current is 2.00 A, how
long does the plating take?
Copyright © Cengage Learning. All rights reserved.
19 | 110
First, we convert mass to moles Cr; then to moles
e-; then, using current, to seconds.
1mol Cr
3 mol e- 96,485 C
1s
1.00 g Cr
52.00 g Cr 1mol Cr 1mol e
2.00 C
= 2.78 × 103 s = 46.4 min
Copyright © Cengage Learning. All rights reserved.
19 | 111
?
A solution of nickel salt is electrolyzed
to nickel metal by a current of 2.43 A. If
this current flows for 10.0 min, how
many coulombs is this?
How much nickel metal is deposited in
the electrolysis?
Copyright © Cengage Learning. All rights reserved.
19 | 112
We first find the charge:
C
60 s
2.43 10.0 min
s
1 min
= 1458 C
Given the half-reaction: Ni2+(aq) + 2e- Ni(s)
1mol e1mol Ni 58.69 g
1458 C
96,485 C 2 mol e
1mol Ni
= 0.443 g Ni
Copyright © Cengage Learning. All rights reserved.
19 | 113