Cooling Tower
advertisement

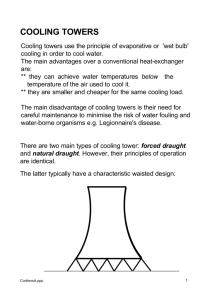
King Saud University College of Engineering Chemical Engineering Dept. Chemical Engineering Laboratory ChE 403 Meshal Khaled Al-Saeed 423105653 Dr. Malik Al-Ahmad Introduction The cooling tower is one of the most important device in chemical industries for example when the hot water come from heat exchanger we use the cooling tower to cool it. The purpose of cooling tower is to cool relatively warm water by contacting with unsaturated air. The evaporation of water mainly provides cooling. In a typical water cooling water tower, warm water flows countercurrent to an air stream. Typically, the warm water enters the top of packed tower and cascades down through the packing, leaving at the bottom. Air enters at the bottom of the tower and flows upward through the descending water. The tower packing often consists of slats of plastic or of packed bed. The water is distributed by troughs and overflows to cascade over slat gratings or packing that provides large interfacial areas of contact between the water and air in the form of droplets and films of water. The flow of air upward through the tower can be induced by the buoyancy of the warm air in the tower (natural draft) or by the action of a fan. The water cannot be cooled below the wet bulb temperature. The driving force for the evaporation of the water is approximately the vapor pressure of the water less the vapor pressure it would have at the wet bulb temperature. Theory Overall Mass Balance: In Put = Out Put L2 + G1 = L1 + G2 → (1) Water Mass Balance: L2 - L1 = G2 * H2 - G1 * H1 → (2) L2 - L1 = G * (H2 - H1) → (3) Energy Balance: Q = G * (HG2 - HG1) → (4) G1 = * air → (5) G2 = G1 * H2 → (6) HG = Cs * (T - To) + o * H → (7) Cs = Cp Liquid + Cp Vapor * H → (8) To determine the number of transfer unit (NTU): H G2 d HG NTU H Gi H G H G1 To calculate height of transfer unit (HTU): G HTU K G a.M B .P Z d Z Z (HTU) (NTU) 0 To calculate the mean driving force: ΔH LM Simpson’s rule: x2 H G2 H G1 H G2 ln H G1 h x f(x)dx 3 [f(x o ) 4f(x 1 ) f(x 2 )] o Where: L2: Flow rate of water in [kg/s.m2]. L1: Flow rate of water out [kg/s.m2]. G1: Air in [kg/s.m2]. G2: Air out [kg/s.m2]. : Volumetric flow rate of air. H: Humidity of air [kg water/kg air]. HG: Enthalpy of the air [J/kg air]. KGa: Mass transfer coefficient of air [kg mol/s.m3.Pa]. NTU: Number of transfer unit [dimensionless]. HTU: Height of transfer unit [m]. Schematic Apparatus Figure 1: Photo of cooling tower. Figure 2: Schematic apparatus for cooling tower. Results Table1: Temperature at Q = 1.0 kW Time (min) Air in Air out Water T1 (ºC) T2 (ºC) T3 (ºC) T4 (ºC) T5 (ºC) T6 (ºC) 5 21 19 22 22 31 23 10 21 19 20 21 29 22 15 21 19 20 21 29 21 20 21 19 20 21 29 21 Flow rate of water = 40 g/sec Initial pressure = 31 mm H2O Final pressure = 38 mm H2O Pressure drop = 7 mm H2O Volume of evaporation water at 20 min = 1027 ml Table2: Temperature at Q = 1.5 kW Time (min) Air in Air out Water T1 (ºC) T2 (ºC) T3 (ºC) T4 (ºC) T5 (ºC) T6 (ºC) 0 22 19 22 22 31 21 5 21 19 23 24 32 22 10 21 19 24 24 32 21 15 21 19 24 25 32 22 20 21 19 24 25 33 22 Flow rate of water = 40 g/sec Initial pressure = 31 mm H2O Final pressure = 38 mm H2O Pressure drop = 7 mm H2O Volume of evaporation water at 20 min = 1025 ml Where: T1: Dry bulb temperature in. T2: Wet bulb temperature in. T3: Dry bulb temperature out. T4: Wet bulb temperature out. T5: Water temperature input. T6: Water temperature output. Conclusion From conclusion point view: Cooling tower is used to cool relatively hot water. As the humidity of the inlet air decreased, the performance of the cooling tower will be better. This leads to the better mass transfer between water and gas phase. As the temperature of the inlet air decreased, the performance of the cooling tower will be better. As the temperature increased overall mass transfer coefficient KGa increased. If the air flow rate is increased, the height of the cooling tower decrease. Recommendations It is better to open the windows or doors in the lap to refresh the air and to make a good deference in the driving force. Summary The main objective of this experiment is to perform mass and energy balances over a cooling tower and to determine the mean driving force, the number of transfer units and the overall mass transfer coefficient. In this experiment can be calculated: The outlets water for a typical cooling tower and compare it with the measured value. The rate of heat transfer. The mean driving force. Also can be get HGi from the Temperature Enthalpy diagram and the operating line then get the number of transfer unit (NTU) by determine the area under the curve then find the overall mass transfer coefficient. Results: At Q = 1.0 kW L1 = 0.04 kg/s Q = 0.524 kW NTU = 0.285 HTU = 1.68 m ∆HG Lm = 49.23 kJ/kg dry air KGa = 2.9 e-6 kg mol/s.m2 At Q = 1.5 kW L1 = 0.0401 kg/s Q = 1.25 kW NTU = 0.0879 HTU = 5.523 m ∆HG Lm = 60.39 kJ/kg KGa = 9.73 e-7 kg mol/s.m2 References 1. Chirstie J. Geankoplis, "Transfer Process and Unit Operation", 3rd edition. 2. Dep. of Chemical Eng “Chemical Engineering Laboratory II Manual”. 3. Perry’s, "Chemical Engineers Handbook", 5th edition. 4. From web side: “ http://www.armfield.co.uk/ ”