Experiment II: Steam Distillation: Isolation of Limonene from Orange
advertisement

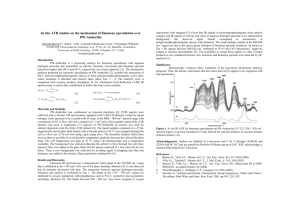
Experiment II: Steam Distillation: Isolation of Limonene from Orange Peels Purpose. To extract out and characterize a simple organic compound (a terpene) from oranges via steam distillation. Discussion. The distillation of a homogeneous binary mixture produces a vapor to which each component in the mixture contributes a part. The distillation of a heterogeneous binary mixture, on the other hand, produces a vapor mixture that is only dependent upon the temperature. Thus, if a mixture is composed of water and a high-boiling, water-immiscible substance, the mixture will boil near but somewhat below 100oC. Most of the distillate will be water, but a separable portion of it will be the immiscible component, usually in a high state of purity. The process just described, called steam distillation, is an excellent technique for the isolation of many water-insoluble compounds that are unstable at temperatures near their boiling points, or compounds difficult to isolate or purify in any other way. Steam distillations may be carried out in the following ways. The compound to be separated and water are simply placed in a conventional distillation apparatus and the distillation collected. This is called direct steam distillation. The distillate, which appears as a heterogeneous mixture, may be either two liquid layers or a solid and water. A separation is easily effected by way of the separatory funnel or by simple separation. In the present experiment we will isolate by way of direct steam distillation a sample of limonene from a few oranges. Limonene (see IR spectrum below for structure) is an essential oil made up of two isoprene units, , to form one of a class of compounds known as terpenes. Experimental Procedure. Estimated time of experiment: 1.5 h. While it is always good experimental practice to use a chemical fume hood, this experiment may be done outside of the hood. Physical Properties of Components* Compound Literature Density value Literature Refractive index (RI) Water Limonene *Note: See question #2 (below) for filling in this chart. The apparatus for this experiment has been assembled for you and will be placed at your laboratory station. With the help of a high-speed food blender, prepare a puree from the parings of four large navel oranges (see note 1). Use sufficient distilled water to produce approximately 300 mL of slurry (add a bit of water at a time; it is easier to add water to dilute than to peel more oranges to add more orange parings). Place all of the orange peel slurry in the distillation flask and bring mixture to a steady boil with a properly adjusted heater setting. Avoid violent boiling, for excessive frothing results and may carry small amounts of slurry directly into the condenser. Should this occur, the contents of the receiver must be returned to the distillation flask, the condenser flushed with fresh distilled water, and the distillation resumed more judiciously. Periodically add 5-10 mL distilled water to the distillation flask from the separatory funnel to maintain a constant volume. Continue the distillation until about 25 mL of condensate has been collected. By this time and oily layer may be seen on the surface of the collected distillate and about 1 mL of the top oily layer should be present. If no oily layer appears, it may be necessary to collect a larger volume of condensate or to recollect some limonene, the distillation may be stopped and any water remaining in the separatory funnel discarded. Collect the top layer via a Pasteur pipet. If the limonene does not separate out in the graduated cylinder, transfer the distillate to the separatory funnel and separate the organic layer from the water. Store the limonene collected in a clean test tube or vial for IR analysis. Note 1. A citrus grater or sharp knife can be used to remove only the outer portion of orange peel. The heavy white pulp which constitutes the bulk of the orange peel is not to bee used. The citrus grater generally gives excellent yields. Characterization. 1. Obtain an IR spectrum of your sample of limonene and compare it to that shown in Figure (1). Attach the IR spectrum to your notebook pages on this lab. 2. Obtain the RI of your sample of limonene and compare it to the literature. Record the number in your notebook pages on this lab. Figure 1 Sample: limonene %T _X_ ABS ___ Sample: _liquid_ 16 scans Matrix material: _neat_ limonene Questions: (to be answered neatly in your laboratory notebook) 1. The terpene, squalene, is a natural organic compound originally obtained for commercial purposes primarily from shark liver oil. How many isoprene units make up this compound? The structure of squalene is: 2. During the lab, fill in the densities of water and limonene as well as the RI’s of these compounds using the Aldrich catalog. (If this catalog hasn’t been shown to you yet, see your instructor). You will use this reference book throughout the lab course.