3 - Chemistry Test 1 Study Guide - Hopewell - Chemistry
advertisement

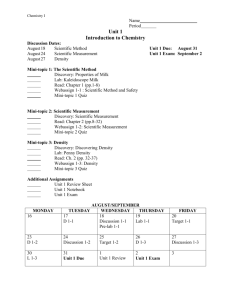
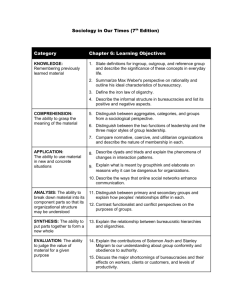
Chemistry Test 1 Study Guide Chapters 1-3 Chapter 1 - 5 Fields of Chemistry: Organic, Inorganic, Analytical, Physical, Biochemistry Scientific Method - Experiment Law - Chemistry Matter - Independent Variable Observation - Dependent Variable Theory - Hypothesis Chapter 2 Scientific Notation Powers of 10 – what they are and how to complete different mathematical operations Significant Digits Accuracy vs. Precision (think of the dart boards) Percent error SI Units o Length = meter (m) o Mass = kilogram (kg) use gram (g) for your base unit when converting o Volume = Liter (L) remember that 1 mL = 1 cm3 o Temperature = Kelvin (K), Celsius (oC) K = oC + 273 o Energy = Joule Metric Prefixes o Kilo103 o Deci10-1 o Centi10-2 o Milli10-3 o Micro10-6 o Nano10-9 Dimensional Analysis (Conversions) o Multiply the # you are trying to convert, by a conversion factor. o Conversion factors are equalities, such as 1ft=12in, or 60sec=1min. o Write the conversion factor as a fraction, with the unit you want to cancel out in the denominator. Ex. Convert 5 hours to seconds 5hrs x 60 min x 60 sec = 18, 000 sec 1 hr 1 min Density = mass volume units: g cm3 or g mL Chapter 3 Distinguish between states of matter (chart in your notes- definite vs. indefinite) Distinguish between physical and chemical properties. Distinguish between physical and chemical changes. - Know several signs of a chemical change Classify matter –Pure Substances (elements and compounds) -Mixtures (homogeneous and heterogeneous) Distinguish between extensive (the extent of matter) and intensive properties (sample size does NOT matter) Know how to separate different types of separation (filter, evaporation, distillation, etc.) Review vocab on pages 21, 49, 81 – no need to memorize, just be familiar with it Questions: 1) Give the rules for: a) Adding and subtracting significant figures. b) Multiplying and dividing significant figures. 2) How do solids, liquids and gases differ? (think about the chart in your notes) 3) Which of the following are physical properties? Hardness of steel taste of coffee Flammability of gasoline Elasticity of rubber conductivity of copper a rotting banana Smell of perfume solubility of salt in water 4) Which of the following are chemical changes? Fermenting grapes rusting iron Evaporation of dry ice Forming a snowflake Dissolving salt in water cooking bread dough electricity flowing through a metal decomposing water into hydrogen and oxygen 5) Which of the following substances are homogeneous? An ice cube a can of paint Motor oil(used) scrambled eggs piece of wood a container of salt freshly opened ginger ale newspaper 6) Label the following as compound of element Sulfur dioxide Glucose iodine oxygen sand silver salt ammonia water calcium chloride