Term 3 - Quiz 1- 2011 / 2012
advertisement
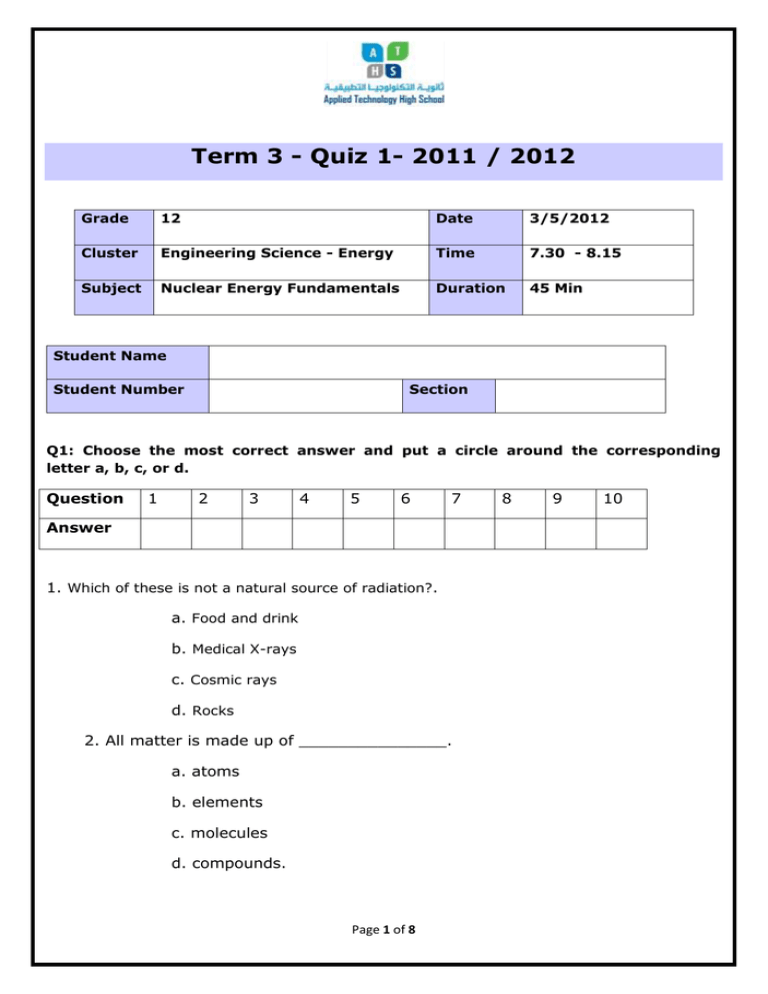
Term 3 - Quiz 1- 2011 / 2012 Grade 12 Date 3/5/2012 Cluster Engineering Science - Energy Time 7.30 - 8.15 Subject Nuclear Energy Fundamentals Duration 45 Min Student Name Student Number Section Q1: Choose the most correct answer and put a circle around the corresponding letter a, b, c, or d. Question 1 2 3 4 5 6 7 Answer 1. Which of these is not a natural source of radiation?. a. Food and drink b. Medical X-rays c. Cosmic rays d. Rocks 2. All matter is made up of _______________. a. atoms b. elements c. molecules d. compounds. Page 1 of 8 8 9 10 3. The number of ________________ inside the nucleus is called the atomic number. a. protons b. neutrons c. electrons d. nucleus 4. The number of protons and _______________ are always equal in a neutral atom. a. protons b. neutrons c. electrons d. nucleus 5. The ___________ number is the number of protons and neutrons. a. atomic b. mass c. electron d. nucleus 6. _________________ is the name of the scientist who performed the Gold Foil experiment. a. Thomson b. Dalton c. Rutherford d. Aristotle. 7. __________________ have the same atomic number but different mass number. a. Atoms Page 2 of 8 b. Elements c. Isotopes d. chemicals. 8. What sort of reaction has happened when hydrogen atoms become a helium atom a. Chemical reaction. b. Ionic reaction. c. Nuclear reaction. d. None of the above. 9. What does not happen when a nucleus splits? a. Nuclear fusion b. Radiation is released c. New nuclei are formed d. New elements are formed 10. Given the diagram representing a reaction. Which phrase best describes this type of reaction and the overall energy change that occurs? a. Nuclear, and energy is released b. Chemical, and energy is released c. Chemical, and energy is absorbed d. Nuclear, and energy is absorbed Q2: Referring to the picture of Rutherford’s Gold Foil experiment shown below, write down Rutherford’s observations and conclusions? Use the table below to write your answers. Page 3 of 8 Observation Conclusion ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ ______________________________ a. Explain Radioactive decay of carbon -14. ………………………………………………………….. ………………………………………………………….. ………………………………………………………….. ………………………………………………………….. ………………………………………………………….. Page 4 of 8 Q3: Refe rring to the figur e sho wn, ………………………………………………………….. b. What is meant by the substance’s half –life? ………………………………………………………………………………………………………………………. ……………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………….. Q4: Name the following diagram and the indicated parts. Select your answers from the list provided below. Hot coolant out Cold coolant in Nuclear fuel Movable control rod Reactor vessel Graphite core Concrete shield Nuclear reactor Page 5 of 8 1 2 7 7 3 4 5 6 6 a. The diagram name is: ________________________________________ b. Fill in the indicated parts. Write your answers in the table below. Number Name (select from the list) 1 2 3 4 5 6 7 Q5: Given the following reaction: a) State the type of the above reaction. Page 6 of 8 __________________________________________________________ b) Describe the reaction. __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ c) State two disadvantages of utilizing the heat generated by this reaction in electrical power generation. __________________________________________________________ __________________________________________________________ __________________________________________________________ __________________________________________________________ Page 7 of 8 Page 8 of 8