Summer Chemistry Final Review (2 nd Semester)
advertisement

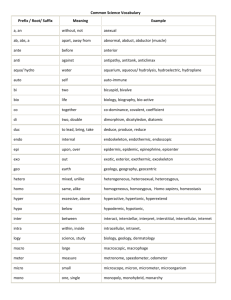
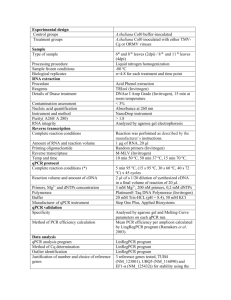
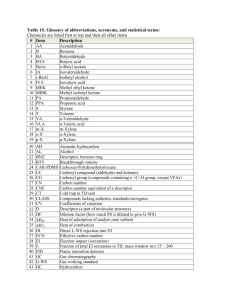
Summer Chemistry Final Review (2nd Semester) Multiple Choice (105 Q’s) (4 choices) 1.) What do symbols in a chemical rn mean? E.g. (s), (l), (g), (aq), , , , , +, , etc… 2.) What do symbols in a chemical rn mean? E.g. (s), (l), (g), (aq), , , , , +, , etc… 3.) Understanding reactant verses products. E.g. reactants products. 4.) What do symbols in a chemical rn mean? E.g. (s), (l), (g), (aq), , , , , +, , etc… 5.) The meaning of a catalyst. 6.) Balancing a chemical equation. 7.) Balancing a chemical equation. 8.) Understanding the Laws of: Definite Proportions, Multiple Proportions, Conservation of Mass. 9.) Balancing a chemical equation. 10.) What determines if a Single Replacement Reaction will occur? 11.) What determines if a Single Replacement Reaction will occur? 12.) What are the reactants and products of a Combustion Reaction? 13.) Determine the products of a Double Replacement Reaction, then balance. 14.) What are the reactants of a Double Replacement Reaction? 15.) What are the reactants and products of a Combustion Reaction? 16.) Identify the type of reaction. E.g. Synthesis, DeComp, Single & Double Replacement & Combustion. 17.) Identify the type of reaction. E.g. Synthesis, DeComp, Single & Double Replacement & Combustion. 18.) Identify the type of reaction. E.g. Synthesis, DeComp, Single & Double Replacement & Combustion. 19.) Identify the type of reaction. E.g. Synthesis, DeComp, Single & Double Replacement & Combustion. 20.) The meaning of Avogadro’s number. 21.) What does Atomic Mass mean? 22.) What does Atomic Mass, Molar Mass and Formula Mass mean? 23.) What does 22.4 L represent? 24.) What does STP mean and what are their values? 25.) What did Avogadro say about # moles and Volumes of gas at the same Temp & Pressure? 26.) What does percent composition mean? 27.) What does Empirical formula mean? 28.) Determine which formula is an Empirical Formula? 29.) Which pair of formulas have the same Empirical Formula? 30.) What happens to mass during a chemical reaction? 31.) Determine the mole to mole ratio from a balanced chemical equation. 32.) Calculation: Mole to mole. 33.) Calculation: Volume to Volume. 34.) Calculation: Volume to Volume. 35.) What is the meaning of Limiting & Excess Reactants (aka Reagents)? 36.) What is the meaning of Limiting & Excess Reactants (aka Reagents)? 37.) What is the meaning of Actual Yield, Theoretical Yield & Percentage Yield? 38.) What are the 3 ways to speed up the dissolving process? 39.) What to the amount you can dissolve if you double, triple or halve the amount of water? 40.) What is the relationship between Solubility and the Temperature of the water? 41.) What does saturated, concentrated and dilute mean? 42.) Calculation: What is the Molarity of a Solution given #moles and Volume of water? 43.) What changes (and does not change) when you dilute a solution? 44.) How is % by volume represented? 45.) Calculation: Find V(Solute). 46.) What are the 5 characteristics of Acids, and Bases? 47.) Know the formulas of common acids. 48.) What are the 5 characteristics of Acids, and Bases? 49.) What are the 5 characteristics of Acids, and Bases? 50.) What is the formula for the Hydrogen ion, Hydroxide ion or Hydronium ion? 51.) What’s an Arrhenius Acid and Arrhenius Base? 52.) What’s an Arrhenius Acid and Arrhenius Base? 53.) What is the meaning of Mono, Di & Tri Protic? 54.) Given a concentration, determine if the solution is acidic or basic (alkaline) e.g. [OH-] = 10-11M is acidic or basic? 55.) What does self ionization of water mean? 56.) What determines if a solution is neutral? 57.) Given a concentration, determine if the solution is acidic or basic (alkaline) e.g. [H+] = 10-4M is acidic or basic? 58.) What is the meaning of pH? 59.) What is the pH range? What values are acidic, and basic? 60.) Given a concentration, determine if the solution is acidic or basic (alkaline) e.g. [H+] = 10-4M is acidic or basic? 61.) What are some strong acids, weak acids? What are some strong Bases, weak bases? 62.) What are the 5 properties of an ideal gas? 63.) What is the relationship between Pressure and Volume? 64.) What is the relationship between # moles and Pressure? 65.) How does a change in temperature affect the gas molecules? 66.) Boyles, Charles, Gay Lussac’s, Combined and Ideal Gas Laws. What quantities do each involve? 67.) Boyles, Charles, Gay Lussac’s, Combined and Ideal Gas Laws. What quantities do each involve? 68.) What is the relationship between Pressure and Volume? 69.) What is the relationship between Pressure and Temperature? 70.) Boyles, Charles, Gay Lussac’s, Combined and Ideal Gas Laws. What quantities do each involve? 71.) Boyles, Charles, Gay Lussac’s, Combined and Ideal Gas Laws. What quantities do each involve? 72.) Boyles, Charles, Gay Lussac’s, Combined and Ideal Gas Laws. What quantities do each involve? 73.) Boyles, Charles, Gay Lussac’s, Combined and Ideal Gas Laws. What quantities do each involve? 74.) Calculation: Partial Pressures. 75.) What is the relationship between velocity and mass of different gases? 76.) What is the meaning of elastic (and inelastic) collisions? 77.) What are the 5 properties of an ideal gas? 78.) What are the pressure measuring instruments and what do they measure specifically? 79.) What are the SI units of Force and Pressure? 80.) What does STP mean and what are their values? 81.) What happens to atmospheric pressure at high altitudes, and below sea level? 82.) At absolute zero, what happens to the motion of particles? 83.) What is the relationship between temperature and kinetic energy? 84.) What is a fluid? 85.) What is the relationship between evaporation and temperature? 86.) Which molecules in a liquid evaporate? 87.) What is the relationship between evaporation and temperature? 88.) What is the relationship between evaporation and temperature? 89.) What is the meaning of Dynamic Equilibrium? 90.) What is the relationship between evaporation and temperature? 91.) What happens to the liquid when its vapour pressure matches the atmospheric pressure? 92.) What does normal boiling point mean? 93.) What happens to boiling temperature at high altitudes, and below sea level? 94.) Compare the melting temperatures of ionic solids versus molecular solids. 95.) Properties of solids. 96.) The parts of a crystal lattice. 97.) The meaning of melting, boiling, evaporation, condensation, sublimation… 98.) The meaning of melting, boiling, evaporation, condensation, sublimation… 99.) The meaning of melting, boiling, evaporation, condensation, sublimation… 100.) Identifying parts of a Phase Diagram. 101.) Identifying parts of a Phase Diagram. 102.) Identifying parts of a Phase Diagram. 103.) Identifying parts of a Phase Diagram. 104.) Identifying parts of a Phase Diagram. 105.) Identifying parts of a Phase Diagram. Calculations Section: (10 Q’s) These are calculations that are solved via algebra (a formula) or through the Chain method. But there are 4 multiple choice answers to choose from. 106.) Calculate the Molar Mass of a substance. 107.) Molecules to mass (not switching substances) calculation. 108.) Grams to moles (switching substances). 109.) Calculate Molarity given mass (in g), Molar Mass and Volume (in mL). 110.) Changing concentrations. Calculate the new concentration (Molarity) of a diluted solution. 111.) Calculate the pH of a diprotic acid solution. e.g. pH of 2.5x10-6M H2CO3 solution. 112.) Charles Law calculation. 113.) Mass (g) to Volume (L) (switching substances) calculation. 114.) Ideal Gas Law calculation. 115.) Specific Heat calculation.