1_1.4_quicklime & slaked lime - science
advertisement

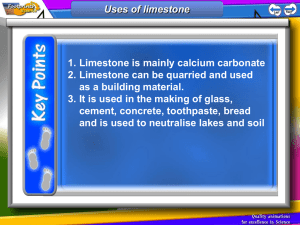
Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Practice these equations in FULL – you choose the level of difficulty; 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Thermal decomposition of magnesium carbonate Calcium added to hydrochloric acid Zinc metal added to sulphuric acid Calcium oxide is added to water to form a single compound Thermal decomposition of calcium carbonate Zinc metal added to hydrochloric acid Calcium is added to water Magnesium metal is burnt Hydrogen gas is added to chlorine gas Calcium is added to oxygen gas Magnesium metal is added to hydrochloric acid GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. MgCO3 (s) (in heat forms) MgO (s) + CO2 (g) Ca (s) + 2HCl (l) (forms) CaCl2 (s) + H2 (g) Zn (s) + H2SO4 (l) (forms) ZnSO4 (l) + H2 (g) CaO (s) + H20 (l) (forms) Ca(OH)2 (l) CaCO3 (s) (in heat forms) CaO (s) + CO2 (g) Zn (s) + 2HCl (l) (forms) ZnCl2 (l) + H2 (g) Ca (s) + H20 (l) (forms) CaO (s) + H2 (g) 2Mg (s) + O2 (g) (forms) 2MgO (s) H2 (g) + Cl2 (g) (forms) 2HCl (l) 2Ca (s) + O2 (g) (forms) 2CaO (s) Mg (s) + 2HCl (l) (forms) MgCl2 (l) + H2 (g) GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Key words; lime water, mortar, slaked lime GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Learning Objectives: By the end of the lesson I can: • Describe what slaked lime is and how it is produced. Quicklime (calcium oxide) reacts with water to produce slaked lime (calcium hydroxide). •Explain the processes involved to make lime mortar. Mortar can be made by mixing slaked lime (calcium hydroxide) with sand and water. • Limestone and its products have many uses, including slaked lime, mortar, cement and glass. • Atoms and symbols are used to represent and explain what is happening to the substances in chemical reactions. GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide What’s wrong with this sentence? Limestone is a metamorphic rock and is made of the pure element, calcium carbonate. •Limestone is a sedimentary rock •It is mainly made up of calcium carbonate •Calcium carbonate is a compound •It is actually a mixture of substances GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide What is the chemical & common names for each substance? CaCO3 Chemical: Calcium Carbonate CaO Chemical: Calcium Oxide Ca(OH)2 Chemical: Calcium Hydroxide CO2 Chemical: Carbon dioxide Common: Limestone/Chalk/Marble Common: Quicklime Common: Slaked Lime (or Limewater when in solution) Common: Carbon dioxide GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Limestone Cycle: GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Limestone Cycle: When limestone (calcium carbonate) is heated strongly, it decomposes to give solid quicklime (calcium oxide) and carbon dioxide gas. When a small amount of water is added to quicklime it gives slaked lime (calcium hydroxide). If a lot of water is added to the slaked lime a solution of lime water or calcium hydroxide is formed. If carbon dioxide is bubbled through lime water, limestone is formed again, along with water. GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Limestone Cycle: Carbon Dioxide Lime water Quicklime Water Slaked lime GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Aim: To investigate the limestone cycle Equipment: The • Gauze, Tripod, bunsen burner & heat proof mat Limestone • Tongs, glass rod, pipette, straw Cycle • 2xboiling tubes, test tube rack • Filter funnel & filter paper • Calcium carbonate, water Method: 1. Place a piece of CaCO3 on the gauze mat & strongly heat the edge until it has been white/orange hot for a few minutes. What type of reaction have you just completed? BE SAFE: CaO holds it heat for a long time & are basic –harmful 2. Using tongs, transfer the CaO to a boiling tube, add afew drops of water & observe. What happened? 3. Fill the boiling tube till its 1/3 full and shake gently. BE SAFE: When water is added to CaO it will spit – so be sure to be wearing goggles! 4. Filter the mixture and keep the filtrate. What is the name of the filtrate? 5. Submerge a straw into the filtrate & gently blow. If the solution turns cloudy then the cycle has been completed successfully. Well Done! Add observations, word & symbol equations to your limestone cycle diagram GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Common Name: QUICKLIME Chemical Name: CALCIUM OXIDE Molecular formula: CaO Calcium carbonate calcium oxide (s) + carbon dioxide (g) CaCO3(s) CaO (s) + CO2 (g) Common Name: SLAKED LIME Chemical Name: CALCIUM HYDROXIDE Molecular formula: Ca(OH)2 Calcium oxide (s) + water (l) calcium hydroxide (s) CaO (s) + H2O (l) Ca(OH)2 (s) Common Name: LIMEWATER Molecular formula: Ca(OH)2 (aq) Chemical Name: CALCIUM HYDROXIDE IN SOLUTION Calcium hydroxide (s) + water (l) calcium hydroxide (s) Ca(OH)2 (s) + H2O (l) Ca(OH)2 (aq) Common Name: LIMESTONE Chemical Name: CALCIUM CARBONATE Molecular formula: CaCO3 Calcium hydroxide (aq) + carbon dioxide (g) calcium carbonate (s) + water (l) Ca(OH)2 (aq) + CO2 (g) CaCO3 (s) + H2O (l) GCSE Core Chemistry Exam tip; Make sure you can write balanced equations for the reactions of quicklime with water & slaked lime with carbon dioxide Learning Objectives: By the end of the lesson I can: • Describe what slaked lime is and how it is produced. Quicklime (calcium oxide) reacts with water to produce slaked lime (calcium hydroxide). •Explain the processes involved to make lime mortar. Mortar can be made by mixing slaked lime (calcium hydroxide) with sand and water. • Limestone and its products have many uses, including slaked lime, mortar, cement and glass. • Atoms and symbols are used to represent and explain what is happening to the substances in chemical reactions. GCSE Core Chemistry