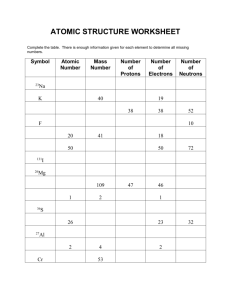
Chem Extra Chp 4 Practice
advertisement
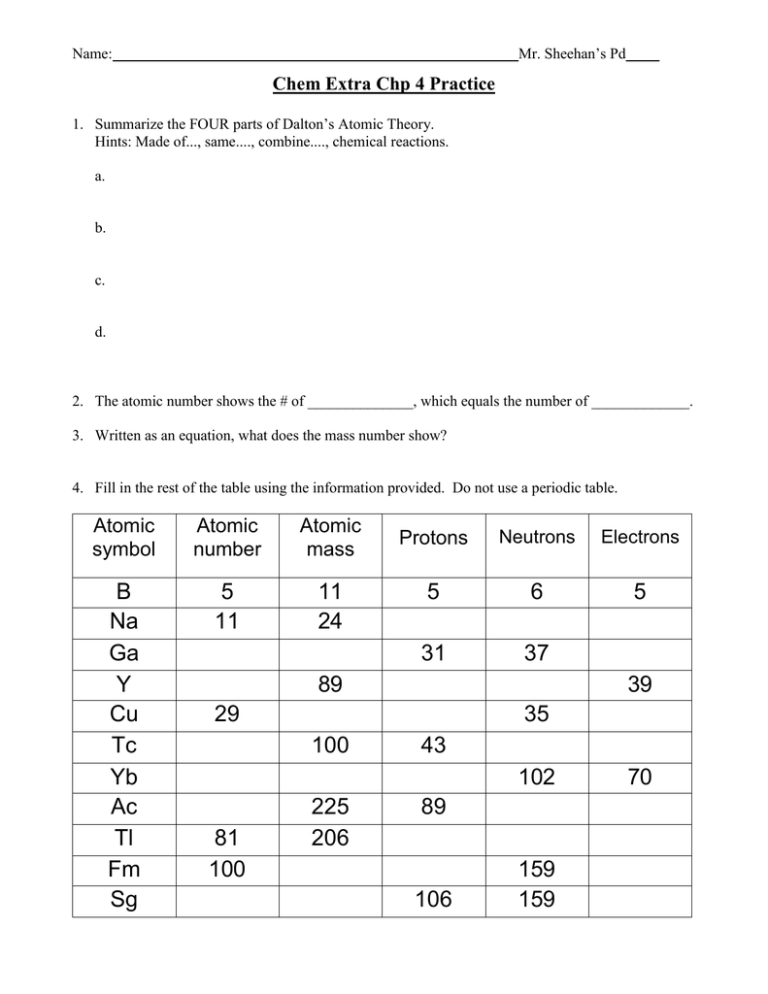
Mr. Sheehan’s Pd Name: Chem Extra Chp 4 Practice 1. Summarize the FOUR parts of Dalton’s Atomic Theory. Hints: Made of..., same...., combine...., chemical reactions. a. b. c. d. 2. The atomic number shows the # of ______________, which equals the number of _____________. 3. Written as an equation, what does the mass number show? 4. Fill in the rest of the table using the information provided. Do not use a periodic table. Atomic symbol Atomic number Atomic mass B Na Ga Y Cu Tc Yb Ac Tl Fm Sg 5 11 11 24 Protons Neutrons Electrons 5 6 5 31 37 89 39 29 35 100 43 102 81 100 225 206 89 106 159 159 70 Mr. Sheehan’s Pd Name: 5. Fill in the table given the element. Round the atomic mass to the nearest whole number. Atomic symbol Atomic Mass Neutrons Protons Electrons number Number 6. Find the average atomic mass of the problems below: ***How do you find average atomic mass? a. The relative abundance and atomic masses of copper isotopes are 69.2% for mass = 62.93 amu, and 30.8% for mass = 64.93 amu. Calculate the average atomic mass of copper. b. Calculate the atomic mass of bromine. The two isotopes of bromine have atomic masses and relative abundance of 78.92 amu (50.69%) and 80.92 amu (49.31%) c. Neon has two isotopes. Neon with 19.992 amu is 90.00% abundant and the mass of 21.99 amu is 10.00% abundant. Calculate the average atomic mass of neon. d. What is the atomic mass of silicon if 92.21% of its atoms have mass 27.977 amu, 4.70% have mass 28.976, and 3.09% have mass 29.974 amu?