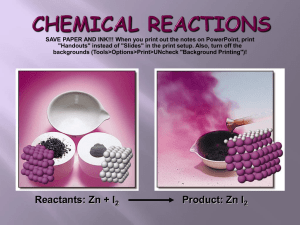
Elements vs. Compounds
advertisement

Name: _____________________________________ Per: __________ Elements vs. Compounds Part 1: Identify the following as an element, compound or diatomic element using the letters E, C or DE: Li ______ Ba ______ CaCl2 ______ H2 ______ O2 ______ FeBr2 ______ Mg(NO3)2 ______ H2O ______ Fe ______ K ______ Cl2 ______ CH4 ______ Na2O ______ List all of the diatomic elements that exist: __________________________________________________ Part 2: Determining how many atoms of each element are in a given chemical formula. Type of Compound No subscripts Example NaCl Only subscripts H2SO4 Subscripts and parentheses Mg(NO3)2 How many total elements? How many atoms of each element? Practice: Chemical Formulas # of Elements # of atoms of each element 1. FeO ______________ ______________________________________ 2. IF5 ______________ ______________________________________ 3. Al2(SO4)3 ______________ ______________________________________ 4. AuCl3 ______________ ______________________________________ 5. K3AsO4 ______________ ______________________________________ 6. Ca(C2H3O2)2 ______________ ______________________________________ 7. (NH4)2CO3 ______________ ______________________________________ 8. Cs2HPO4 ______________ ______________________________________ 9. CuSO3 ______________ ______________________________________ 10. BeSO4 ______________ ______________________________________ Homework: # of Elements # of atoms of each element F3NO3S ______________ ______________________________________ CH3OCH3 ______________ ______________________________________ (CH3)2C2O4 ______________ ______________________________________ (C2H5)2NH ______________ ______________________________________ Ca(C2H3O2)2 ______________ ______________________________________ Cu(NbO3)2 ______________ ______________________________________ Mg3(Si2O5)(OH)4 ______________ ______________________________________ Describe in your own words, the difference between elements and compounds. ___________________________________________________________________________________________ ___________________________________________________________________________________________ ___________________________________________________________________________________________