Instructions on how to name a Covalent
advertisement

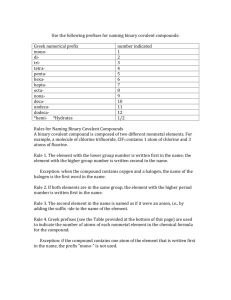
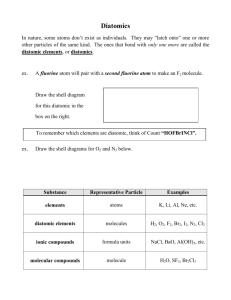
Instructions on how to name a Covalent (aka Molecular) compound: remember that they are made of 2 non-metals non-metals can form a wide range of compounds using the same 2 elements For example: NO, N2O and NO2 are all made of N and O so we CAN’T call them all nitrogen oxide Because of this, we need to name them a different way, so we use Greek Prefixes: 1 = mono 5= penta 9= nona 2= di 6= hexa 10= deca 3= tri 7= hepta 4 = tetra 8= octa STEPS to naming a covalent compound: 1. Look at first Element in formula and determine # of atoms, if it’s greater than one, use corresponding greek prefix infront of element name. If it’s one, just use element name 2. Look at second element in formulas and determine # of atoms for all #’s of atoms, use corresponding greek prefix infront of element name then add “ide” to the end of the name. Examples: N2O CF4 P2O3 BUT!! What if it’s just one element??? Certain NON-metal elements are diatomic (which means that they form a bond with themselves)… you’ll have to memorize these: I Br Cl F O N H You can remember these by the following Sentence “I Bring Chlorine For Our New Hottub” So, if a question asks you for a formula for Oxygen , it’s O2 (oxygen is on the list of diatomic molecules) but if it asks for Phosphorous, it’s just P (Phosphorous is NOT on the list of diatomic molecules)