Journal of Physical and Chemical Reference Data
advertisement
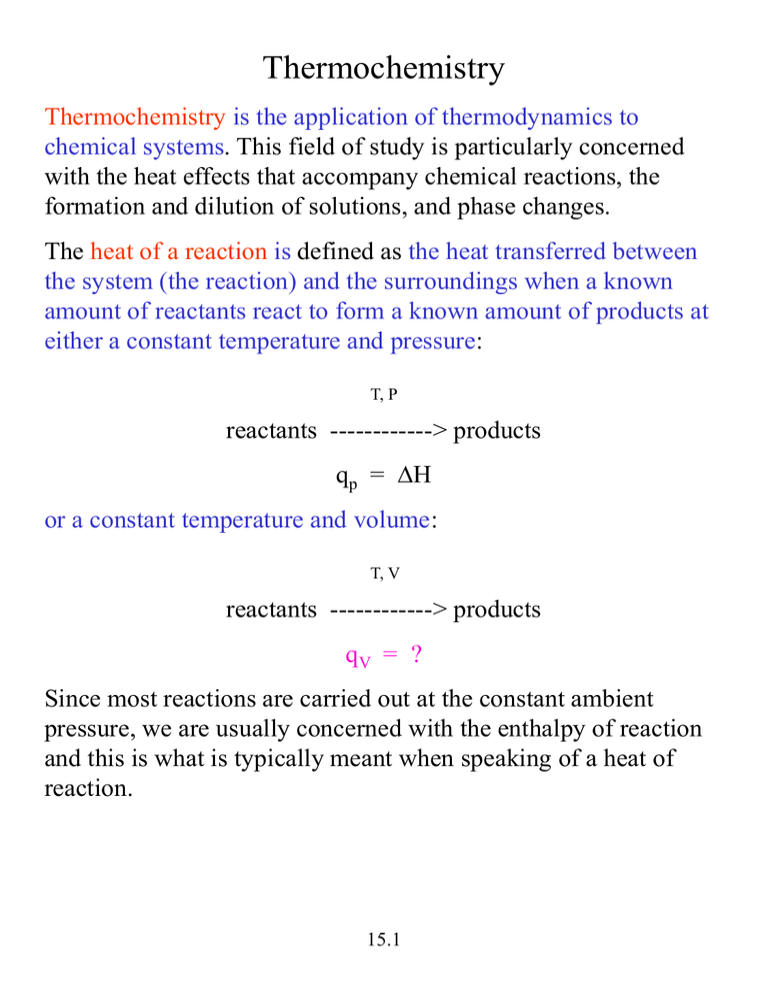
Thermochemistry Thermochemistry is the application of thermodynamics to chemical systems. This field of study is particularly concerned with the heat effects that accompany chemical reactions, the formation and dilution of solutions, and phase changes. The heat of a reaction is defined as the heat transferred between the system (the reaction) and the surroundings when a known amount of reactants react to form a known amount of products at either a constant temperature and pressure: T, P reactants ------------> products qp = DH or a constant temperature and volume: T, V reactants ------------> products qV = ? Since most reactions are carried out at the constant ambient pressure, we are usually concerned with the enthalpy of reaction and this is what is typically meant when speaking of a heat of reaction. 15.1 There are between 10 and 20 million known compounds that can actually or hypothetically react with each other in an astronomical number of ways and it is therefore literally impossible to catalog all the possible heats of reaction. To get around this problem we define for each substance a standard reaction and tabulate its associated heat of reaction. These reactions and their associated heats of reaction can then be used to calculate the heats of other reactions. This standard reaction is known as the standard formation reaction of some substance and is defined as the balanced reaction in which 1 mole of that substance (the only product) is formed from the elements which comprise it (the reactants) in their elemental standard states at 1 bar of pressure and some temperature (usually, but not necessarily 25.0 oC). In older tables the standard pressure was defined as 1 atm, rather than 1 bar. How significant is this change in the definition of standard pressure? For example, the standard formation reaction for the interhalogen, iodine monobromide, is: 298 K, 1 bar 1/2 I2 (s) + 1/2 Br2 (l) ------------> 1 IBr (s) The heat associated with the standard formation reaction is known as the standard heat of formation for IBr (s) and is: DHof, 298 K [IBr (s)] = - 10.5 kJ/mole of IBr (s) The superscript, o, indicates standard state conditions, e.g., that the pressure is 1 bar, while the subscript, f, indicates that this is a formation reaction. 15.2 This is a page taken from the National Technical and Information Service (NTIS) formerly the National Bureau of Standards (NBS) publication The NBS Tables of Chemical Thermodynamic Properties, Wagman. et. al., eds., Journal of Physical and Chemical Reference Data, Vol 11 (Supp. No. 2), 1982. Substance Molar mass Formual and Description State ICI -1 g mol o 0K o DfH 0 298.15 K (25 C) and 0.1 MPa (1 bar) o o o o o H -H 0 S DfH DfG -1 -1 kJ mol -1 kJ mol Cp -1 J mol K g ao 162.3574 162.3574 162.3574 19.41 --- 17.78 ---13.8 -5.46 -17.1 -8.8 9.548 ----- 247.551 --152.7 35.56 ----- ICl ICl2 g ao 162.3574 197.8104 992.9 --- 997.5 --- ---161.0 ----- ----- ----- ICl3 cr 233.2634 --- -89.5 -22.29 --- 167.4 --- ao cr g ao 289.2618 206.8314 206.8314 206.8314 --49.79 ----- -137.7 -10.5 40.84 --- -116.3 --3.69 --- ----9.904 --- 221.3 --258.773 --- ----36.44 --- in CCl4:x + - I2Cl IBr Note: ao implies a 1 molal aqueous solution in which the substance is not ionized, ai implies that it is ionized cr implies the crystalline or solid state What do you think g stands for? Note further that the data has energy units of either kJ or J and that the standard pressure is 1 bar. Finally note the units on S and Cp are different from the units on DH and o DG , etc. o o Why are there dashes, ---, for some of the entries? For which thermodynamic variable is the greatest amount of data present? 15.3 The reactants in the formation reaction are the elements in their elemental standard state, i.e., the most stable form of the element at a pressure of 1 bar and the specified temperature (again this is usually, but not necessarily 25 oC). At 1 bar and 25 oC only a few elements are gases. All the Nobel gases are monatomic gases: He (g), Ne (g), Ar (g), Kr (g), Rn (g) while the remaining elemental gases are all diatomic: H2 (g), N2 (g), O2 (g), F2 (g), Cl2 (g) Even fewer elements are liquids at 1 bar and 25 oC: Br2 (l), Hg (l), and perhaps Fr (l) ? All the remaining elements are solids at 1 bar and 25 oC. Many solid elements can occur in several different crystal phases or allotropes at 1 bar and some specified temperature, say 25 oC, and only the most stable of these crystalline phases represents the elemental standard state. For example elemental sulfur can adopt 10 different allotropic forms, but it is the rhombohedral crystalline phase that is stable at 1 bar and 25 oC and is therefore the elemental standard state of sulfur at this temperature. What do you think the elemental standard state of carbon is at 25 oC, diamond or graphite? What do you think the elemental standard state of astatine, At, is at 25 oC? 15.4 With this definition it should be clear that the standard enthalpies of formation of the elements in their standard states are zero: 298 K, 1 bar Ni (s) ----------> Ni (s) DHof, 298 K [Ni (s)] = 0 The standard enthalpy of formation of copper(II) sulfate pentahydrate, CuSO4x5H2O (s), at 25 oC is: DHof, 298 K [CuSO4x5H2O (s)] = - 2279.7 kJ / mole Write the balanced standard formation reaction associated with this value. 15.5 Standard enthalpies of formation tabulated at some temperature can be used to calculate the standard enthalpy change for some reaction at that same temperature. The procedure is to sum the molar standard enthalpies of formation of each product scaled by the coefficients in the balanced reaction for each product and from this sum over the products subtract a similar sum over the reactants, where the molar standard enthalpy of formation of each reactant is also scaled by the coefficient in the balanced reaction for that reactant. This verbose procedure is best illustrated by an example: Fossil fuels contain sulfur and when these fuels are burned one of the products is sulfur dioxide, SO2 (g). An important reaction in the formation of acid rain involves the reaction of sulfur dioxide, with molecular oxygen, O2 (g), to form sulfur trioxide, SO3 (g), which can subsequently react with water droplets, H2O (l) to form sulfuric acid, H2SO4 (aq): 298 K, 1 bar 2 SO2 (g) + O2 (g) -----------> 2 SO3 (g) The standard enthalpy change for this reaction at 25 oC can be calculated from the standard enthalpies of formation of all the reactants and products as: DHo298 K = +(2 mols SO3) DHof, 298 K [SO3 (g)] - (2 mols SO2) DHof, 298 K [SO2 (g)] - (1 mol O2) DHof, 298 K [O2 (g)] 15.6 Substituting tabulated values of the standard enthalpies of formation gives: DHo298 K = +(2 mols SO3) (- 395.72 kJ / mole SO3) - (2 mols SO2) (- 296.830 kJ / mole SO2) - (1 mol O2) ( 0 kJ / mole O2) = - 197.78 kJ Note that this result can be viewed as having units of moles in the denominator, since we can interpret the result as: = - 197.78 kJ / 2 moles of SO3 formed or = - 197.78 kJ / 2 moles of SO2 reacted or = - 197.78 kJ / 1 moles of O2 reacted Using data from the National Technical and Information Service (NTIS) calculate the standard enthalpy change, ΔHo, at 25 oC for the combustion of 1 mole of octane, C8H18 (l) in the presence of excess oxygen? C8H18 (l) + 25/2 O2 (g) -------------> 8 CO2 (g) + 9 H2O (l) Also calculate the standard internal energy change, ΔEo, for this reaction? 15.7 The use of standard enthalpies of formation to calculate the standard enthalpy change for some reaction is a specific example of a more general procedure known as Hess’s law (it’s not really a law) that states that if you have a set of reactions that add to give a net reaction, then thermodynamic values for this set of reactions add in the same way to give the corresponding thermodynamic value for the net reaction. Hess’s law is usually applied to reaction enthalpies. The direct conversion of graphite to diamond is not easily carried out (or we would all be doing it) and hence it is also not easy to measure the enthalpy change associated with this phase change. It is, however, fairly straight forward to burn (combust) either graphite or diamond (there may be some tear shedding as we part with our diamond) in the presence of exess oxygen and measure the associated heats of combustion: C (gr) + O2 (g) -------> CO2 (g) DHo298 K = - 393.5 kJ C (d) + O2 (g) -------> CO2 (g) DHo298 K = - 395.4 kJ Note that, if we reverse the 2nd reaction, the two reactions will add to give a reaction describing the conversion of graphite to diamond: C (gr) + O2 (g) -------> CO2 (g) CO2 (g) -------> C (d) + O2 (g) ----------------------------------------C (gr) ---------> C (d) DHo298 K = - 393.5 kJ DH 298 K = - (- 395.4 kJ) -------------------------------DHo298 K = + 1.9 kJ o Note that when adding reactions, equal numbers of moles of the same species in the same phase that occur in both reactants and products cancel. Any reactants or products that don’t cancel end up as reactants or products in the net reaction. Note also that reversing a reaction, changes the sign on the enthalpy change. 15.8 When solid metallic copper is dropped into concentrated nitric acid, copious noxious dark brown fumes of nitrogen dioxide are produced: 3 Cu(s) + 8 HNO3(aq) + O2(g) ---> 3 Cu(NO3)2(aq) + 2 NO2(g) + 4 H2O(l) The reaction of the solid copper with the nitric acid actually initally produces nitric oxide: 3 Cu (s) + 8 HNO3 (aq) --> 3 Cu(NO3)2 (aq) + 2 NO (g) + 4 H2O (l) which immediately reacts with molecular oxygen in the air to produce the nitrogen dioxide: NO (g) + 1/2 O2 (g) -----> NO2 (g) DHo298 K = - 57.07 kJ / mole In addition to the above data, use the following standard enthalpies of formation: DHof,298 K [H+ (aq)] 0 by definition DHo,f,298 K [NO3- (aq)] = - 205.0 kJ / mole DH o f,298 K [Cu2+ (aq)] = + 64.77 kJ / mole DHof,298 K [NO (g)] = + 90.25 kJ / mole DHof,298 K [H2O (l)] = - 285.83 kJ / mole to calculate the heat of the overall reaction initially described in this problem. Note that you may have to scale all the coefficients in one or more of these reactions by some constant scaling factor and, if you do this, the enthalpy change for that reaction is also scaled by the same factor. 15.9 Use the following data: DHo298 K, combustion [1-hexene] = - 4003 kJ / mole DHo298 K, combustion [hexane] = - 4163.12 kJ / mole to determine the enthalpy change for the hydrogenation of 1-hexene to hexane: CH3(CH2)3CH=CH2 (l) + H2 (g) -------> CH3(CH2)4CH3 (l) You may need to look some data up in your textbook. 15.10







