Assessment 11 Renal I
advertisement

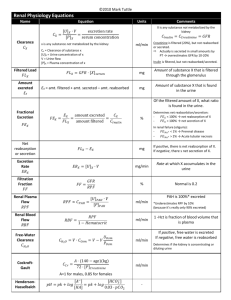
Renal Professor Hints Assessment 11 1/12/11 Calculating fractional excretion of sodium, potassium; Assessing clearance ratios; Calculation of Plasma anion gap and urinary anion gap. 𝐺𝐹𝑅 = [𝑈𝑟𝑖𝑛𝑒𝑐𝑟𝑒𝑎𝑡𝑖𝑛𝑖𝑛𝑒 ] 𝑥 𝑈𝑟𝑖𝑛𝑒 𝐹𝑙𝑜𝑤 𝑅𝑎𝑡𝑒 (𝑚𝑙/𝑚𝑖𝑛) [𝑃𝑙𝑎𝑠𝑚𝑎𝑐𝑟𝑒𝑎𝑡𝑖𝑛𝑖𝑛𝑒 ] Inulin could also work (freely filtered), but creatinine is used more in the clinical setting since it estimates being freely filtered (even though it is slightly secreted) 𝐶𝑙𝑒𝑎𝑟𝑎𝑛𝑐𝑒𝑥 = [𝑈𝑟𝑖𝑛𝑒𝑥 ] 𝑥 𝑈𝑟𝑖𝑛𝑒 𝐹𝑙𝑜𝑤 𝑅𝑎𝑡𝑒 (𝑚𝑙/𝑚𝑖𝑛) [𝑃𝑙𝑎𝑠𝑚𝑎𝑥 ] 𝐶𝑙𝑒𝑎𝑟𝑎𝑛𝑐𝑒 𝑅𝑎𝑡𝑖𝑜𝑥 = 𝐶𝑙𝑒𝑎𝑟𝑎𝑛𝑐𝑒𝑥 𝐶𝑙𝑒𝑎𝑟𝑎𝑛𝑐𝑒𝑐𝑟𝑒𝑎𝑡𝑖𝑛𝑖𝑛𝑒 (𝑜𝑟 𝑖𝑛𝑢𝑙𝑖𝑛) 𝐶𝑙𝑒𝑎𝑟𝑎𝑛𝑐𝑒 𝑅𝑎𝑡𝑖𝑜𝑥 = If CR > 1.0 then secretion of x is occurring, if CR<1.0 it suggests that reabsorption is occurring. If CR is 0 it is either too large to be filtered, or it is completely reabsorbed (glucose, amino acids). 𝑅𝑒𝑛𝑎𝑙 𝑃𝑙𝑎𝑠𝑚𝑎 𝐹𝑙𝑜𝑤 = 𝐶𝑙𝑒𝑎𝑟𝑎𝑛𝑐𝑒𝑥 𝐺𝐹𝑅 [𝑈𝑟𝑖𝑛𝑒𝑃𝐴𝐻 ] 𝑥 𝑈𝑟𝑖𝑛𝑒 𝐹𝑙𝑜𝑤 𝑅𝑎𝑡𝑒 (𝑚𝑙/𝑚𝑖𝑛) [𝑃𝑙𝑎𝑠𝑚𝑎𝑃𝐴𝐻 ] 𝐹𝑖𝑙𝑡𝑟𝑎𝑡𝑖𝑜𝑛 𝐹𝑟𝑎𝑐𝑡𝑖𝑜𝑛 = 𝐺𝐹𝑅/𝑅𝑃𝐹 FF usually equals 20% 𝑃𝑙𝑎𝑠𝑚𝑎 𝐴𝑛𝑖𝑜𝑛 𝐺𝑎𝑝 = 𝑁𝑎 – (𝐶𝑙 + 𝐻𝐶𝑂3) 𝑈𝑟𝑖𝑛𝑎𝑟𝑦 𝐴𝑛𝑖𝑜𝑛 𝐺𝑎𝑝 = (𝑁𝑎 + 𝐾)– 𝐶𝑙 Urinary Anion Gap o Indirect estimate of NH4 in urine o Clinical labs can’t measure NH4 easily in the urine, therefore labs measure the urinary anion gap and estimate NH4 indirectly o Sodium and potassium are the major urinary cations (we must consider potassium in urine, don’t necessarily have to consider it with plasma anion gap since it has a small plasma concentration) o Chloride is the major urinary anion Not bicarbonate o Urinary anion gap is about 10 mEq/L normally Can vary widely depending on meal states, etc. o This is useful when you are trying to evaluate normal anion gap metabolic acidosis (usually hyperchloremic since it’s metabolic acidosis) 𝐹𝑖𝑙𝑡𝑒𝑟𝑒𝑑 𝑙𝑜𝑎𝑑 = 𝑃𝑙𝑎𝑠𝑚𝑎𝑥 × 𝐺𝐹𝑅 Essential information: Understand the directional changes that occur in the fluid spaces (total body water, intracellular fluid volume, extracellular fluid volume and its components) during pathophysiologic settings such as volume expansion volume deficiency, dehydration, etc. 2/3 TBW is ICF Renal Professor Hints Assessment 11 1/12/11 1/3 TBW is ECF ¾ ECF is interstium ¼ ECF is plasma volume (NOTE: PLASMA Na tells you nothing about TBNa content or size of ECFV (since it also reflects TBW)!, ECFV is controlled by TBNa) Look at P.102 Volume Depletion (isotonic) Volume Loss (H2O) TBW ↓ ↓ ECFV ↓ ↓ ICFV Unchanged ↓ Isotonic Volume Expansion ↑ Depends on TBNa and TBW relative concentrations Hypotonic Volume expansion ↑ Hypertonic (salt intake)Volume Expansion unchanged ↑ ↑ ↑ (movement into ICFV) ↓ (movement into ECFV) Be able to evaluate an arterial blood gas with attendant electrolytes. Only simple acid-base disorders will be included (i.e., No mixed acid-base disorders). Henderson-Hasselbach Equation: o H+=24 x [Acid (carbon dioxide) /Base (Bicarbonate)] o At normal pH (7.4) the H+ is 40 nEq/L o The only way you can change pH is to change this ratio H+ is usually equal to 80 minus the last two digits of the pH (ex. pH of 7.4, 80 – 40 = 40) Golden Rules o Carbon dioxide and bicarbonate always change in the same direction in all of the four disorders o Secondary mechanisms are always present in a simple disorder (if it is not a mixed disorder) o These mechanisms never fully correct but always bring pH back towards normal 24× (𝐶𝑂2 ) 𝐻+ = Note: If pH=7.40 then (80-40=40nEq/L of H+), therefore 40=[24 x 40 (normal CO2) ] 𝐻𝐶𝑂3 /HCO3, this makes bicarb equal to 24 which is normal for a pH of 7.40 under normal circumstances (way to check yourself) In respiratory orders chloride changes opposite than bicarbonate (think—in respiratory acidosis we have too much CO2too much H+bicarbonate goes down immediately due to buffering and more later due to kidney regulation of bicarbonate) Renal Professor Hints Assessment 11 1/12/11 Utilizing clinical information and measurements of plasma and urinary concentrations you should be able to assess renal handling of sodium, water, and potassium. For example: in a given clinical setting, is the kidney appropriately conserving these solutes and water (or alternatively is there evidence for inappropriate wasting or conservation). Sodium: If you are volume depleted you will be trying to conserve sodium, if you have volume excess you will be giving off sodium. If you see someone who has a huge loss of volume and they are giving off tons of sodium something does not fit here. Water: Differences in DI and Primary Polydipsia Condition Central DI Polyuria Nephrogenic DI + Primary Polydipsia + low (<100) + Uosm low (<100mOsm/kg H2O) Posm high* high* low PNa high* high* low Uosm in response to water deprivation no change (<300) no change (<300) increase (>500) Uosm in response to DDIVP increase little/no change *- usually seen if there is decrease in access to water low (<100) little/no change Potassium: Decreased Secretion (hyperkalemia) Increased Secretion (hypokalemia) Increased movement into cells (hypokalemia) Renal failure Diuretics (metabolic alkalosis) Insulin Distal tubular dysfunction Prolonged vomiting, nasogastric suction (metabolic alkalosis) Epinephrine Recall: Contraction Alkalosis (decreased volume increase aldotherefore increase H+ secretion by alpha intercalated) Aldo also increase K secretion – Increased movements into extracellular fluid (hyperkalemia >5.5meq/L) Blocking insulin (blocks K (via Na/K ATPase) into ICF, but K channel remains open and therefore results in increased ECF K ) Beta antagonists (blocks into ICF, but K channel remains open and therefore results in increased ECF K ) Renal Professor Hints Assessment 11 1/12/11 don’t forget this. hypoaldosteronism Measure urine chloride (should be low in vomiting—due to loss of HCl) Bartter’s syndrome, Gitelman’s syndrome (metabolic alkalosis) Hyperaldosteronism (and hyper renin) This is also a hypertensive hypokalemic disorder (most of the others are hypotensive) Renal Tubular acidosis (metabolic acidosis) Cushings, Congenital adrenal hyperplasia (all working on the aldo receptor (either aldo or its precursors, ie. DOC)increase K secretion via principal cells Increased plasma K (not a cause of hyperkalemia per se but increases movement of K intracellularly) Alkalosis (exchange H into ECF for K into cell) Acidosis (exchange H into cell for K into ECF) Tx. Bicarbonate therefore moves K into cells Albuterol Rapid cell proliferation (think tumor—uses lots of K) Increased Posm shifts fluid from ICF to ECF and K exists with water (solvent drag) Cell lysis EKG Findings with Potassium Disorders ( KNOW ABOUT THE U WAVE AND SPIKED T WAVE—NOT JUST FOR ASSESSMENT). Tx. Hypokalemia: Amiloride, Triamterene, sprinolactone, oral or i.V, replacement Tx. Hyperkalemia: insulin, bicarbonate, albuterol Note: changes in plasma sodium mainly determined by changes in total body water Free Water: - in hypotonic urine Cwater is positive as Uosm < Posm Renal Professor Hints Assessment 11 1/12/11 - in isotonic there is no Cwater as Uosm = Posm - in hypertonic urine Cwater is negative as Uosm > Posm Estimation of TBW from sodium - Water excess = 0.6 TBW x (1- [Na]observed/140) - Water deficit= 0.6 TBW x ([Na]observed/140 -1) Proximal tubular disorders: know clinical features of Fanconi syndrome, cystinuria, cystinosis, wilson’s ds, primary renal phosphate wasting syndromes; know normal physiology of prox tubule Defect Clinical Signs Fanconi’s Cystinuria (AR) Cystinosis Wilson’s ds. (AR) Dysfunctional proximal tubule (most likely due to energy producing machinery, ie. Mitochondria, sodium gradient, ATP pathways, etc.) Defect in cystine, ornithine, lysine, arginine transporter (COLA) Cystine stone formation Lysosomal storage disorder Defect in ATPB7 causes increased copper in liver and elsewhere, decreased ceruloplasmin Increased amino acids, glucose, phosphate, bicarbonate, uric acid in urine Presents as a hyperchloremic hypokalemic (since bicarbonate is gone) metabolic acidosis Damages kidney, thyroid, cornea, and retina, growth retardation RTA in kidneys (defect in bicarbonate excretion), causes nephrocalcinosis also Kayser Fleischer rings, basal ganglia tremor, parkinsonism, etc. Causes Wilson’s ds, Lowes, Galactosemia, Tyrosinemia, Hereditary fructose intolerance, Mitochondrial disorders,GSD’s Acquired: Heavy metals, drugs, chemicals, cancer, hyperparathyroidism (phosphate depletion) Normal function of PT: OMO-defect in phosphatonin (FGF23) Bone, dental ds., low calcitriol levels (should be elevated with low phosphate) Tx. With phosphate and calcitriol Calcium and PTH are normal while ALP is elevated Can be seen in both cystinuria and cystinosis Hypophasphatemic Rickets, RTA II (Since bicarbonate is lost) Cystinosis* (common) ADHR-defect in FGF23 Cystine stones Muscle cramps, dehydration, polyuria, polydipsia, fatigue, failure to thrive, and rickets. Increased ALP Associations Primary Renal Phosphate Wasting Syndromes XLH-defect in PHEX Cystine crystals on slit lamp exam Common cause of Fanconi’s Impaired transport of cystine from lysosome into cytoplasm Note: Tx. Of this does not correct Fanconi’s RTA, nephrocalcinosis Renal Professor Hints Assessment 11 1/12/11 Reabsorbs glucose, amino acids, bicarbonate, phosphate Reabsorbs 50% of filtered sodium chloride and water Secretes many compounds, final pathway in synthesis of hormones (ex. Calcitriol) THINK: BULK REABSORPTION Distal tubular disorders: know normal physiology; know clinical features of Bartter syn, Gitelman syndrome, Liddle syn, nephrogenic diabetes insipidus, renal tubular acidosis Defect Bartter’s (AR) Na/K/2Cl pump (NKCC2) and ROMK (potassium back transporter) Think: Loop Diuretic Gitelmans Thiazide sensitive transporter in distal convoluted tubule Liddle Syndrome Gain of function of epithelial sodium channel (opposite of what Amiloride /Triamterene do) NDI Mut. In AQP2 or anything downstream Type I: impaired ammonia secretion in collecting duct (hypokalemic) Can also be mut. in chloride channel or barrtin channel (this is associated with sensineural hearing loss) Clinical Signs Hypochloremic metabolic alkalosis Think: It’s acidosis since there is such a huge loss of volumeincrease RAASincrease K and H secretionbicarb goes up in serum and chloride goes down Hypochloremic metabolic alkalosis Dehydration, salt craving, hypercalciuria (neonatal has nephrocalcinosis but adult form doesn’t), hypokalemia, hyperaldosteronism, elevated PGE2 Hyperplasia of Juxtaglomerular apparatus Tx. Potassium and magnesium supplements Hypochloremic metabolic alkalosis Similar to Bartter’s but has hypomagnesemia and hypermagnesuria Less symptomatic since less sodium is conserved via DCT than in the thick ascending limb ( think a loop vs. a thiazide) Hypertension, hypokalemic metabolic alkalosis RTA Type II: Can’t reabsorb bicarbonate in the PT Inability to concentrate urine in the face of high plasma osmolarity Type IV: deficiency of aldosterone (hyperkalemic) Hyperchloremic Metabolic Acidosis Urine pH in Type I may be 6-7.0, whereas in Type IV and II it is <5.5 Think: Type II is giving off bicarb in urine (can’t reabsorb it). Remember, RTA is a normal anion gap acidosis (always check anion gap in metabolic acidosis) Fractional excretion of bicarbonate (urine bicarb/plasma bicarb all over urine creatinine/plasma creatinine –just and indicator for absorption or secretion) is >1015% in proximal RTA, <5% in distal RTA, <510% in hyperkalemic RTA Positive urinary anion gap in Type I and hyperkalemic RTA, negative during acidosis Renal Professor Hints Assessment 11 1/12/11 due to diarrhea U-B CO2 is low in Types I and IV but normal in Type II Characteristics of RTA Types Finding Plasma K+ Type II (bicarb loss) Low Type I (can’t secrete H) Low Type IV (aldo def.) High Urine pH < 5.5 >5.5 Variable Urine Anion Gap U-B PCO2 Positive Positive Positive Normal Low Low FE HCO3- > 15% < 5% <5-10% Associated Findings Fanconi syndrome Increased UCa2+ Renal insuff. in some TTKG: (Kurine/Kplasma)/(Uosm/Posm), ratio of concentrations of potassium divided by total concentrations—basically uses the serum osmolarity as a baseline to let us know if potassium is being appropriately secreted or reabsorbed relative to total osmolarity. If TTKG<2.0 in the face of hypokalemia then it is appropriate. If TTKG>8.0 in the face of hyperkalemia then it is appropriate. Tells us that the mineralocorticoids are working correctly. Pitfalls: Can’t be used well when sodium is less than 20 mEq/L or urine osmolarity is <300mOsm/kg Question: Patient presents wtih cardiac arrhythmias and a spiked T wave on EKG. The TTKG is 4.3 is this appropriate? Renal Professor Hints Assessment 11 1/12/11 Answer: No, the spiked T wave along with the cardiac arrhythmia points toward hyperkalemia. We would expect a TTKG > 8.0 in hyperkalemia (getting rid of potassium) if everything was working correctly. There perhaps could be an issue with a mineralocorticoid binding, receptors, etc. She said to know these two slides in class. Below are the sites that certain diuretics work on. • Acetazolamide: carbonic anhydrase • Furosemide, bumetanide, ethacrynic + + - acid: Na -K -2Cl cotransporter • Thiazides: Na-Cl cotransporter Renal Professor Hints Assessment 11 1/12/11 In general, be familiar with normal ranges of electrolytes. NORMAL LAB VALUES CHEMISTRIES Na+ Cl+ K HCO3 BUN Cr HEMATOLOGY Hgb WBC Hct Glc Plt Sodium 135 – 145 mEq/L WBC 4.5 – 11.0 x 103 / L Potassium 3.5 – 5.1 mEq/L RBC Chloride 98 – 106 mEq/L Hemoglobin 3.8 – 5.7 x 106 / L 13.5 – 17.0 g/dL Bicarbonate 22 – 29 mEq/L Hematocrit 39 – 50% BUN 7 – 18 mg/dL MCV 80 – 96 mcm3 Creatinine 0.6 – 1.2 mg/dL MCH 27 – 33 pg/cell Glucose 70 – 115 mg/dL MCHC 32 – 36% hgb / cell Calcium 8.4 – 10.2 mg/dL Platelets Phosphate 2.7 – 4.5 mg/dL RDW 150 – 400 x 103 / L 11.0 – 16.0% Magnesium 1.3 – 2.1 mg/dL Segs (Neuts) 35 – 73% Anion gap 7 – 16 mEq/L Lymphocytes 15 – 52% Osmolality NH3 275 – 295 mOsm/kg 10-35mMol/L Monocytes 4 – 13% Eosinophils 1 – 3% Protein 6.0 – 8.0 mg/dL Basophils 0 – 1% Albumin 3.5 – 5.5 g/dL Reticulocyte count 0.5-1.5% Total bilirubin 0.2 – 1.0 mg/dL URINALYSIS Dir. bilirubin 0.0 – 0.2 mg/dL Color <yellow> Lipase 10 – 140 U/dL Turbidity <clear> Amylase 25 – 125 U/dL Specific Gravity 1.003 – 1.035 SGOT/AST 7 – 40 U/L pH 4.5 – 8.0 SGPT/ALT 7 – 40 U/L Ketones <negative> GGT 9 – 50 U/L Protein <negative> AlkPhos 38 – 126 U/L Blood <negative> Uric Acid 2.0 – 6.9 mg/dL Glucose <negative> LDH 120 – 240 U/L Nitrite <negative> Free T4 0.71 – 1.85 ng/dL Leukocyte Esterase <negative> 0.32 – 5.00 IU/mL 20-60 mg/dl Osmolality 50 – 1400 mOsm/kg Sodium 40 – 220 mEq/day 2.3-3.5 g/dL Potassium 25 – 125 mEq/day pH 7.35 – 7.45 COAGULATION PaCO2 35 – 45 mmHg PT 12.3 – 14.2 sec PaO2 80 – 100 mmHg PTT 25 – 34 sec HCO3- 21 – 27 mEq/L Bleeding Time 2 – 7 min O2 Saturation 95 – 98% Thrombin Time 6.3 – 11.1 sec Base Excess 2 mEq/L Fibrinogen 200 – 400 mg/dL TSH Ceruloplasmin Globulin ARTERIAL BLOOD GASES Iron TIBC Ferritin 31-184 mcg/dL 235-434 mcg/dL 10-320 ng/mL Folate B12 7-24 ng/mL 200-1000 pg/mL Renal Professor Hints Assessment 11 1/12/11 Hey Everybody: I know we are all feeling a little elated and pressured all at the same time but I just wanted to send a quick note about the renal pathophysiology review. Not many people attended the review yesterday and I had assumed it would be the standard review of past questions too but it was not. It was actually a review but only lasted about 30-40 minutes. Dr. Wall stated the following: Know high yield topics from the module pages 102-108 Know the lecture on renal syndromes pages 446-463 (I believe this was covered in the last review very well too). Know urine analysis results and interpretation. 1. He covered briefly TBW and total body Na+. 2. He also covered simple acid base: metabolic, acute and compensated respiratory acidosis/alkalosis, plasma Anion Gap. 3. There will be at least one GFR question know how creatinine clearance relates to muscle mass and indications. 4. He covered UA routine findings and indications 3+. protein. RBC and red cell casts: inflammatory versus not, acute versus chronic. 5. He mentioned again renal syndromes lecture (pp 446-463, problems 21-24). 6. Know Urine anion gap and its implications ( if high e.g. 10 indicates kidneys have a problem) think RTA. 7. He also said to know where in the kidneys the different diuretics work, loops, hydrochlorothiazide, etc. 8. Know the common glomerular diseases. There will be basically 1 question per contact hour and but he did comment that Renal Syndromes and Urine analysis are probably OVER represented.