Molar Mass of Compound
advertisement

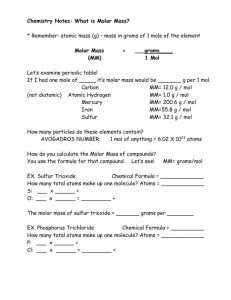
THE MOLE The Mole an amount of substance. ll Intro to Mole Video Pull Intro to the Mole Candy Activity Part 1 - Particles What is a mole? standard counting unit in chemistry (abbreviated “mol”) Relates # particles, mass, and volume of gases YOUR NEW BEST FRIEND THE MOLE CIRCLE!!! PARTICLE PLACE Relates the mole to the number of particles CONVERSION FACTOR: 1 MOLE = 6.02 x 1023 Particles MOLES AND PARTICLES 6.02 X 1023 is also known as Avogadro’s Number Named after Italian chemist Amadeo Avogadro who determined that at the same temp and pressure, all gasses contained the same number of particles Examples of Particles (Amount): Charged Particles Ions Atoms Li+, Ni2+, S2-, N3PO43-, OH-, NH4+ Particles of Ionic Compounds Formula Units Molecules NaCl, CaSO4, Fe(OH)3, AgNO3 What is a formula unit? Molecular Compound CO2 Molecules Formula Unit NaCl Na+Cl-Na+Cl-Na+ClCl-Na+Cl-Na+Cl-Na+ Na+Cl-Na+Cl-Na+Cl- smallest whole # ratio of ions in the cmpd. How many molecules are in 1 mole of molecules? Pull 6.02 x 102 molecules How many formula units are in 1 mole of formula units? Pull How many atoms are in 1 mole of atoms? Pull How many ions are in 1 mole of ions? Pull 1 new conversion factor YOU need to know. 1 mole = 6.02 x 1023 particles Mole Circle!!!! Particle Place SO....back to Dim Analysis Every problem must include for given, conversion factors and answer: Example: 6.02 x 1023 atoms Fe Number Substance Unit Use units - they must cancel Answer in correct significant figures Solving Mole Problems 1. Identify and label Given and Unknown. 2. Write down given x __________. 3. Put units of given on bottom of first conversion factor. 4. Use mole circle to figure out where you are starting, where you are going, how many steps and conversion factors to get you there. 5. Set up using dimensional analysis - number, unit, substance for each entry. Make sure units cancel along the way. 6. Multiply all items in numerator. Divide by numbers in denominator. 7. Answer - sig figs, units. SO....back to Dim Analysis How many atoms are in 3.4 mol of barium atoms? 2.05 x 1024 atoms 3.4 mol 6.02 x 1023 atoms 1 mol = 2.05 x 1024 atoms And... How many mols of iron 5.6 x 1022 iron atoms? are in 0.93 mol Fe 5.6 x 1022 atoms 1 mol 6.02 x 1023 atoms = 0.093 mol How many oxygen atoms are in 2.2 mol of BaCO3? 3.97 x 1024 O atoms 2.2 mol BaCO3 6.02 x 1023 f.u. BaCO3 1 mol BaCO3 3-O atoms 1 f.u. BaCO3 = How many carbon atoms are in a mixture of 2.0 mol of CO2 and 3.5 mol of C6H12O6? 2.0 mol CO2 6.02 x 1023 CO2 molecules 1 mol CO2 1 C atom = 1 CO2 molecule 1.2 x 1024 C atoms 3.5 mol C6H12O6 6.02 x 1023 C6H12O6 molecules 1 mol C6H12O6 1.26 x 1025 C atoms + 1.2 x 1024 C atoms 1.26 x 1025 C atoms 1.38 x 1025 C atoms 6 C atoms 1 C6H12O6 molecule = Intro to the Mole Candy Activity Part 2 - Mass MASS AVE. Do you know how to measure out 1 mole of sugar or salt or water? The mole is a counting unit, so we would have to count out 6.02 x 1023 particles of each substance. (NO THANKS!) There are 2 ways to measure out a number of moles of a substance: Measure it in GRAMS ( a mass) If a GAS, measure it in LITERS ( a volume) MASS AVE. Relates the mole to the mass of one mole of substance CONVERSION FACTOR: 1 MOLE = Molar Mass of Substance Molar Mass/Atomic Mass/Gram Formula Mass/Molecular Weight TAKE OUT YOUR PERIODIC TABLE :) Molar Mass Pull M F Molar mass Pull The mass of 1 mole of substance. Pull Atom Atomic Mass Carbon 12.011 Mg 24.03 Au 196.97 1 mol of Carbon = 12.011 g 1 mol Mg = 24.03 g 1 Mol Au = 196.97 g Pull my Finger Hey that's a NEW conversion factor! 1 mol = molar mass of substance Molar Mass of a compound Pull Simply find the molar mass of each atom and add them together. What is the molar mass of NaCl? Na = 23 g Pull C +l = 35.5 g 58.5 g 1 mole of NaCl has a mass of 58.5 g Pull What is the molar mass of K2SO4? Pull What is the molar mass of Ca(OH)2? Pull 1 mol Ca = 40g x 1 = 40g 1mol O = 16g x 2 = 32g 1mol H = 1g x 2 = 2g + 74g Mole to Mass and Mass to Mole Problems (same process as mole to particles, particles to mole!) How many grams of Al are in 3.00 moles of Al? Answer = 80.94 = 80.9 g Al What is the mass in grams of 2.7 mol of O2? Answer = 86.4 = 86 g O2 Determine the number of moles in 5.00 g of H2O. Answer = 0.277469 = 0.277 mol of H2O Multi-Step Problems - Use the Mole Circle! Everything MUST go through the Mole!!! Calculate the number of grams of Na present in 3.00 x 1018 atoms of Na: = 0.0001146 g or 1.146 x 10-4 g of Na Calculate the number of atoms of Na in 160.00 grams of Na: = 4.189 x 1024 atoms of Na Penicillin, the first of a now large number of antibiotics, has the formula C14H20N2SO4. Calculate the mass of 2.0 x 1010 molecules of penicillin. Molar mass C14H20N2SO4 = 312.4 grams 2.0 x 1010 molecules of penicillin = 1.0 x 10-11 grams of penicillin Molar mass C14H20N2SO4 = 312.4 grams 2.0 x 1010 molecules 1 mol 6.022 x 1023 molecules 312.4 grams 1 mo l = 1.04 x 10-11 grams of penicillin (0.5 mg) (3) = 1.5 mg molar mass caffeine = 194.2 grams 1.5 mg 1g 1 mol 6.022 x 1023 molecules 1000 mg 194.2 g 1 mol = 4.65 x 1018 molecules of caffeine How many atoms of carbon would be in 53.5 grams of isopentyl acetate (C7H14O2) ? Percent Composition Pull % by mass of elements in a compound. Mass of the Part x 100 = % comp Mass of Element Mass of the Whole Pull Molar Mass of Compound To solve: Assume 1 mole of compound. can use..... Then you Find the % composition of water. 1- Determine the formula of the compound and list its elements. Divide the molar mass 2g H element by the of each = 0 .11g 18g molarH2O mass of the entire cmpd. 3- 0.11g x 100 = 11% H 0.89g x 100 = 89% O Find the % composition when 8.20g of Mg combines with 5.40g of O. + 8.20g Mg 5.40g O 13.60g MgO 8.20g Mg 13.60g MgO x 100 5.40g O 13.60g MgO x100 = 60.3% = 39.7% Mg O Empirical Formulas Pull Examples of Empirical Formulas H2O H3PO4 CH H2O2 C6H12O6 Not necessarily the "actual" formula! Molecular Formulas Pull Cmpd. Empirical Formula Molecular Formula formaldehyde CH2O CH2O CH2O acetic acid CH2O C2H4O2 C2H4O2 glucose glucose CH2O C6H12O6 How to Calculate Empirical Formulas Pull Step 1. Find the number of moles of each element. Pull (If % is given, Step 2.100g of compound; assume % = grams) Divide number of moles of each element by smallest number of moles. Step 3. Pull (finding ratio of # moles of each) Find the lowest whole number ratio. Write formula with subscripts reflecting ratio. An alkaline battery contains 63.0 % Mn and 37.0% oxygen by mass. Find the empirical formula of the cmpd. An unknown cmpd was analyzed and found to contain 26.56% K, 35.41% Cr, 38.03% O. Find the empirical formula for the compound. Calculating Molecular Formulas Pull Pull Pull Molar mass of unknown molecular formula will be given to you!!! S D m Pull ra Calculating Molecular Formulas 1. The empirical formula of a compound is NO2. Its molecular mass is 92 g/mol. What is its molecular formula? 2. A compound is found to be 40.0% carbon, 6.7% hydrogen and 53.5% oxygen. Its molecular mass is 60.0 g/mol. What is its molecular formula? Calculating Molecular Formulas: Step 1: Find the empirical formula mass. Step 2: Divide molar mass by emp. formula mass. Step 3: multiply answer from step 2 by the empirical formula (how? multiply subscripts!) What is the molecular formula if it's molar mass is 60 g/mol and it's emp. formula is CH4N? mass is expressed in grams Particles are atoms, ions, molecules and formula units. 1 mol = 6.02 x 1023 particles 1 mol = molar mass If you can convert mass to mols you can then convert mols to particles. If you can convert particles to mols you can convert mols to mass. Attachments Mole Circle Link.docx