e - Milwaukie High

AP
Chemistry
Periodicity
valence orbitals: outer-shell orbitals
-- elements in the same group have the same valence-shell electron configuration
-- since valence e
– are involved in bonding, elements within a group have many of the same properties
Sodium and potassium react w /water to produce hydrogen gas.
Development of the Periodic Table
-- few elements appear in elemental form in nature
(Au, Ag, Hg, a few others)
-- most are in combined forms with other elements
-- In 19th century, advances in chemistry allowed more elements to be identified.
Au Ag Hg
1869: Independently, Dmitri
Mendeleev (Russia) and
Lothar Meyer (Germany) published classification schemes based on similarities in element properties.
** Mendeleev used his scheme to predict the existence of undiscovered elements, and so is given credit for inventing the first periodic table.
** D.M.’s guiding principle was…
“atomic mass.”
Dmitri Mendeleev
1834 –1907
Lothar Meyer
1830 –1895
-- 1913: Henry Moseley bombarded atoms with high-energy electrons and measured the frequency of the X rays given off. X ray frequency generally increased as atomic mass increased, but VERY nicely increased.
Henry Moseley
1887 –1915
Graph of
Moseley’s data
Electron Shells
Even before Bohr, the American
Gilbert Lewis had suggested that e
– are arranged in shells.
-- Experiments show that e
– density is a maximum at certain distances from nucleus.
Ar Gilbert Lewis
1875 –1946
Ne
-- no clearly defined boundaries between shells
(shells are diffuse, i.e., “fuzzy”)
He distance from nucleus
r d r
Approximate bonding atomic radii for the elements have been tabulated.
The distance between bonded nuclei can be approximated by adding radii from both atoms.
e.g., Bonding atomic radii are as follows:
C = 0.77 A, Br = 1.14 A
So the approximate distance between bonded C and Br nuclei = 0.77 + 1.14
= 1.91 A
Atomic Radius
As we go down a group, atomic radius… increases.
-principal quantum number increases (i.e., a new energy level is added)
As we go from left to right across the
Table, atomic radius… decreases.
-- effective nuclear charge increases, but principal quantum number is constant more p + , but no new (i.e., farther away) energy levels
Coulombic attraction depends on… amount of charge distance between charges
2+ 2 – 2+ 2 –
1+ 1 – 2+ 2 –
H
+
+
+
–
–
–
As we go , more coulombic attraction, no new energy level, more pull, smaller size
He + + – –
Arrange the following atoms in order of increasing atomic radius: Sr, Ba, Cs
Sr < Ba < Cs
Ionization Energy : the minimum energy needed to remove an e
– from an atom or ion
M(g) + 1 st I.E. M + (g) + e
–
M + (g) + 2 nd I.E. M 2+ (g) + e
–
M 2+ (g) + 3 rd I.E. M 3+ (g) + e
–
Successive ionization energies are larger than previous ones.
-(+) attractive force remains the same, but there is less e
–
/e
– repulsion
The ionization energy increases sharply when we try to remove an inner-shell electron. e.g., For Mg, 1 st IE = 738 kJ/mol
2 nd IE = 1,450 kJ/mol
3 rd IE = 7,730 kJ/mol
(strong evidence that only valence e – are involved in bonding)
As we go down a group, 1 st IE… decreases.
-more e
–
/e
– repulsion and more shielding
Generally, as we go from left to right, 1 st IE…
Exceptions: e.g., B < Be
2p
Be: 1s 2 2s 2
B: 1s 2 2s 2 2p 1
B doesn’t like
Subshells prefer to be either completely filled
OR half-filled.
(easier to remove B’s single 2p e
– than one of Be’s two 2s e – s)
…than any of these.
N: 1s 2 2s 2 2p 3
O: 1s 2 2s 2 2p 4
More stable to have than to have
This e
– 2p is easier to remove…
down a group…
Electron affinity : the energy change that occurs when an e
– is added to a gaseous atom
For most atoms, adding an e
– causes energy to be… released.
eq. for e
– affinity: A + e
–
A
–
Exceptions: noble gases : the added e
– must go into a new, higher energy level group 2 metals : the added e
– must go into a higher-energy p orbital group 15 elements : the added e
– is the first one to double-up a p orbital
The halogens have the most ( –) electron affinities, meaning that they become very stable when they accept electrons. more ( e
–
–) affinity
= more willing to accept an e
–
O
–141
S
–200
F
–328
Cl
–349
Electron affinities don’t vary much going down a group.
Se
–195
Br
–325
Te
–190
I
–295
He
+
Ne
+
Ar
+
Kr
+
Xe
+
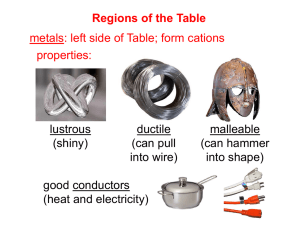
Regions of the Table metals: left side of Table; form cations properties: lustrous
(shiny) ductile
(can pull into wire) good conductors
(heat and electricity) malleable
(can hammer into shape)
-- Because of their low ionization energies, they are often oxidized in reactions.
(i.e., they lose e
–
)
-- Metallic character of the elements increases as we go down-and-to-the-left.
Regions of the Table (cont.) nonmetals: right side of Table; form anions properties: good insulators; gases or brittle solids neon
Ne sulfur
S
8 iodine
I
2
-- memorize the HOBrFINCl twins
(or…Hans and Franz, the ClOBrHIFN twins)
“Wer sind sie?”
“Die ClOBrHIFN Zwillinge!” bromine
Br
2
Regions of the Table (cont.) metalloids (semimetals ): “stair” between metals and nonmetals (B, Si, Ge, As, Sb, Te, Po, At) metals
Si and
Ge computer chips properties: in-between those of metals and nonmetals; “semiconductors”
Si and Ge computer chips
(i.e., a
“basic” oxide)
Reactivity Trends metal oxide + water
MgO(s) + H
2
O(l) metal hydroxide
Mg(OH)
2
(aq) metal oxide + acid
CaO(s) + 2 HNO
3
(aq) salt + water
Ca(NO
3
)
2
(aq) + H
2
O(l) metal + nonmetal
2 Al(s) + 3 Br
2
(l) salt
2 AlBr
3
(s)
(i.e., an
“acidic” oxide)
Reactivity Trends (cont.) nonmetal oxide + water
CO
2
(g) + H
2
O(l) acid
H
2
CO
3
(aq) nonmetal oxide + base
CO
2
(g) + 2 KOH(aq) salt + water
K
2
CO
3
(aq) + H
2
O(l)
Group Trends
Alkali Metals
-- the most reactive metals (one e
– to lose)
-- obtained by electrolysis of a molten salt e.g., chloride ion is oxidized and sodium ion is reduced
2 NaCl(l)
2 Na(l) + Cl
2
(g)
-- react with hydrogen to form metal hydrides:
2 M(s) + H
2
(g) 2 MH(s)
-- react with water to form metal hydroxides:
2 M(s) + 2 H
2
O(l) 2 MOH(aq) + H
2
(g)
-- react w /O
2
: Li yields Li
2
O, others yield (mostly) peroxides (M
2
O
2
)
2 M(s) + O
2
(g) M
2
O
2
(s)
Potassium in water, forming flammable hydrogen and soluble potassium hydroxide.
Alkaline-Earths
-- not as reactive as alkalis (two e
– to lose) compared to alkalis: harder, denser, higher MPs
-- Ca and heavier ones react w /H
2
O to form metal hydroxides
Ca(s) + 2 H
2
O(l) Ca(OH)
2
(aq) + H
2
(g)
-- MgO is a protective oxide coating around substrate Mg
Mg ribbon MgO
Hydrogen
-- a nonmetal, but belongs to no family
-- reacts w /other nonmetals to form molecular (i.e., covalent) compounds
The Hindenburg
Halogens
-At isn’t considered to be a halogen; little is known about it
-- at 25 o C, F
2
Br
2 and Cl is a liquid, I
2
2 are gases, is a solid
-- their exo. reactivity is dominated by their tendency to gain e
–
-- Cl
2 is added to water; the HOCl produced acts as a disinfectant
-- HF(aq) = weak acid;
HCl(aq)
HBr(aq) = strong acids
HI(aq)
A small amount of a halogen is mixed with a noble gas to fill halogen lamps. The halogen sets up an equilibrium with the tungsten filament to prevent the heated tungsten from being deposited on the inside of the bulb.
Noble Gases
-- all are monatomic; have completely-filled s and p orbitals
-- He, Ne, and Ar have no known compounds; Rn is radioactive
-- Kr has one known compoud (KrF
2
);
Xe has a few (XeF
2
, XeF
4
, XeF
6
) professional-grade
Rn detector
Fan for
Rn mitigation
Ionic Radius
Ca atom
20 p +
20 e
–
Ca 2+ ion
20 p
18 e
+
–
Cl atom Cl
– ion
17 p +
17 e
–
17 p
18 e
+
–
Cl Cl
–
Ca Ca 2+
EX. Compare the sizes of Fe,
Fe 2+ , and Fe 3+ .
Then compare Br with Br
–
.
Fe > Fe 2+ > Fe 3+
Br
–
> Br
Electronegativity electronegativity: the tendency for a bonded atom to attract e
– to itself
Electronegativity increases going... up and to-the-right.
Linus Pauling quantified the electronegativity scale.
Most electronegative element is... fluorine (F).
“Oh, man… I forgot which ones the most electronegative elements are.”
F = 4.0
O = 3.5
N = Cl = 3.0
“Shee-oot…
Ow teh ye…
FO’ NCl.”
Others: C = 2.5
H = 2.1