Document
advertisement

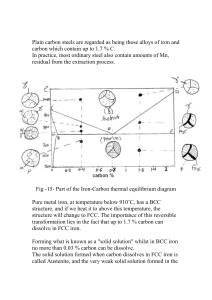
INTRODUCTION Product is an article obtained by transformation of raw material.It fulfill some identified needs and is marketed /sold by the manufacture to eran profit .Hence product is a salable item manufactured in with out compromising for qulity and reliadility. The manufacturing processes help in shaping product with ease at competitive cost.Aproduct can be visualized by a simple input-output model as shown below. Diag.1 The material out which the product is made is known as raw materialand the specific technique used in shape it is known as manufacturing process.Some identified machines and tools are required to shape the product with required dimensional accuracies and finish. The proficiency of individual person in selection of manufacturing process,raw material for product,machines and tools to shape and skill to work on material to shape it matters for a useable product. A product may have single component or may be an assembly of number of components, Services are also been now covered under the definition of product. To be a successful engineer, one has not only know the language of process technologist , but also to practical them, so as to aquire the reasonable knowledge and skill. The manufacturing process can be divided in some of the following groups: 1. 2. 3. 4. Casting Mechanical working of metals (plastic deformation and metal forming) Fabrication Metal machining The raw material used in manufacturing may be metallic and non-metallic, Casting is a process of making metallic items by pouring molten metal in a mould cavity and allowing it cool down. The some of the items made by this process are base and body of machines, Castiron,alluminium , copper base alloys can easily be shaped by the casting process. Mechanical working of the metal is an intentional plasticdeformation of metal , to bring it in desired shape and size under the action of externally applied force . the deformation can be possible at room temperature or elevated temperature. At higher temperature the deformation is quicker and more prominent . Forging is one of the processes of plastic deformation . When work is done using hand tools, the process is known as smith, Chisels,Screw driver, Hammers are some of the items made by smithy or forgong. Except brittle material mechanical working of metals can be used to handle all metals. Fabrication includes cutting, shaping and joining standard section so as to form structure, Bicycle frame, motor cycle chassis, ships etc. are fabricated items. In most of the cased, for joining welding is preferred on account of strong leak proof joint can be made with convenience at low cost. Welding can be defined as the process of joining two metal pieces by heating them to the required welding temperatures, with or without the application of pressure , or use of additional filler materials. Metal machining is a processof shaping material by removing unwanted extra material from the work in the form of small chips. High dimensional accuracles and finishes are the requirement of the product made by assembling two or more components. Such as motor cycle and car engine, electric motors generator etc. Most of the work is done on machine tools and little work by fitting techniques The properties of metals and alloy are considerable improved by controlled heating and coolong of metal in solid state. The process of achieving required properties by heatng and cooling is known as heat treatment, Annealing normalization , hardening etc. are some of the methods of the heat treatment. IIn powder metallurgy componanats are made by metal powder ,On account of high hardness, it is difficult to manufacturing of carbide tool tips or tungsten electrical contacts points. The task is completed by powder metallurgy of powder metallurgy includes making of material powder,pressing powder ,in metallic dies at a very high pressure to get the required shape and strengthening by baking it in a oven maintaining inert atmosphere. Process technologies are backbone for emerging technologies. The achievements of electronics, computer technology, informationtechnology, instrumentation,civil engineering etc. totally based on low cost reliable supporting hardware. Low cost computer is biggest achievement of process technologies is having wide applicationin all the fields. The unlimited capabilities of computer are resulting in simplifying all aspects of product manufacturing .For low cost automation machines are now equipped with industrial computer . The complicity in manufacturing is considerably reduced by computer hardware and software. Diagram 2 Properties of material Selection of material is done based on thierial properties justifying their suitability for the work These properties can be grouped as: 1. 2. 3. 4. 5. 6. 7. Physical properties Chemical properties Electrical properties Magnetic properties Thermal properties Opticaal properties Mechanical properties or Manufacturing properties Physical properties Physical properties are mainly concern with density, porosity, appearance i.e. shape,size and colour etc. They are independent of external force. Chemical properties Engineering materials suffer from chemical deterioration when they come in contact with other substance. Such as corrosion ressistance are considered better.The important chemical properties are: 1. Corrosion resistance 2. Chemical composition 3. Acidity or Alkalinity Chemical properties mainly concern to the structure and composition of a material. Electrical properties Thest propertiesof material are of prime importance for electrical equipments such as electric conductivity, resistivity , di-electric strength etc. Conductivity is the property of the material owing to which the electric current flows easily through the material. The material having resistance for flow of electricity. Magnetic properties These properties are mainly concern to workability of material to shape and performance of material under various loading conditions. They include the elastic and plastic behavior of the material . Important mechanical properties are strength , modulus of elasticity elastic limit, endurance limit, ultimate strength, hardness, brittleness, creep , yield stress, malleability, ducktility, toughnes etc. Mechanical properties of the materials can be determined by loading material under tension, compression, shear, bending, impact etc. Strength Safe load carrying capacity of a material is known as strength of material measured in Kg/cm2 Corresponding to nature of load strength may be tensile, compressive and shear . Universal tensile testing machne , compression testing machine and teting machines are used to measure strength in tension , compression and shear respectively. With application of load deformation is seen in specimen of a material Within certain limits. The material behaves elastically, It comes to original shape and size when load is removed. The limit is known as elastic limit during which the deformation is temprory. The deformation is permanent when load is increased beyond elastic limit . It can take maximum load to ultimate limit and fail/breaks, when further loaded. The resistance offered by the material under the application of the load is known as stress(p) Stress = applied force(F) / area of cross-section (A) = F/A Kg/cm2 The deformation per unit known as strain (e), can be expressed as, change /original dimension . Condering guage length of a specimen under tension. STRAIN = (L1-L2)/L in dimension Where L is guage of unloaded specimen L1 is guage length on deformation HOOKE’S LAW Robert HooKe experimentally verified , that “within elastic limit, stress is directly proportional toth strain”. Stress Stress = constant Strain OR Stress/strain=constant This constant is called as “Modulus of Elasticity” and represented by p E= Where p =stress e e = strain DIAGRAM NO 3 STRESS STRAIN DIAGRAM It’s a graph between stress and strain when load is applied gradually on a test specimen during experimentation. During experimentation data for the change in dimensions in corresponding to applied load.The stress and strain is worked out on the basis of recorded data. A curve is drawn between stress and strain known as stress strain diagram as shown: Diagram no 4 As appearance from the above diagram uner gradual loading condition , the strain is praportionatestress. The point ‘a’ represent the elastic limit of proportionity. The slope of this straight line is known the “modulos or elasticity” or Young’s Modulus, which shows the stiffness of the material . Beyond elastic limit the stress is not proportionate and deformation is fast and permanent. The specimen can take maximum load corresponding to point ‘d’ . The point is known as ultimate point. The little increment in load may result in breaking of test specimen. The deformation is very and specimen breaks at pot e. The strength corresponding yoeld point , ultimate ooint and breaking point is known as yield strength or ultimate strength and breaking strength of material. The percentage elongation can be worked out by measuring the guage length of broken specimen. The shear strength of a material is generally about 50% of its tensile strength and the torsion strength about 75%. In most of the non-ferrous metals it is difficult to locate the yield point. ELASTICITYElasticity if material is the property of regaining original shape after deformation. All the material are elastic, but their degree of elasticity varies with material to material. It’s the desirable property of precisi machine’s and measuring instrument. Steel is said to be a highly elastic material. PLASTICITY Plasticity of the material is the ability of the material to resust deformation . The plastic material can be easily shaped . Under the application of the force. STIFFNESS Stiffness is the property of the material to resist deformation . property helps in retaining shape in the case of the beams, coulumns and component of machine tools. DUCTILITY It is the ability of the material to drawn out under the application of tensile force. Ductile material can be drawn in the form of wires. The material having percentage of elongation more than 15% are said to be a ductile material. BRITTLENESS The material which breaks easily under the application of the load without showing appricible elongation . It is known as Brittle material and this property of material is known as brittleness. Brittleness is the property opposite to that of the Ductility. The material having percentage less than 5% are said to be a brittle material. HARDNESS Hardness can be defined as the resistance to penetration . Material said to be hard when no impressior is seen when a hard point is pressed on a material surface. Hardness of the material can be measured by Vickers, Brinell hardness number Rockwell hardness number . It is the desirable property for cutting tools, High carbon steel, High speed steel, carbidres are hard materials are used for making cutting tools. MALLEABILITY It is the property of material to undergo the great change in shape under compressive loads, Malleable materials such as wrought ion, copper silver etc. can be easily shaped in the form of sheets and other rolled section in hot and cold condition. TOUGHNESS It is the capability of the material to take impact load. Toughness of material is measured by means of charpy or lzod impact testing machine. WEAR Wear can be defined as the progressive loss or the removal material from a surface. Wear generally alerts a part’s surface topography and may result in severe surface damage. RESILIENCE It is the ability of the material to store energyand resist shocks and impact. The material used of making springs need to have resilience. CREEP It is the property of material due to which the material is progressively deformed at a slow rate when subjected to constant stress at elevated temperature for a very long time, as in the case of steam turbine blades. INDURANCE AND FATIGUE STRENGTH Fatigue is the phenomenon thatleads to failure when a material is subjected to cyclic loading results in variation in amount and nature of stresses. The component fails with the initiation of crack on cyclic loading. METAL STRUCTURE Atom Arrangement in Metals Based on arrangement of atoms the materials are classified as crystalline or amorphous.The most of the metals have the crystalline structure in which atoms are arranged in a regular geometric pattern are known as lattices.Lattices are unit building black that is essentially repeated throughout space in a repetitive manner. These block are known as unit cells.Arrangement of the atoms has a significant effect on the material properties. Dig.5 Dig.6 S.No phase Important properties Frrite Ferrite is the grain or crystal of sold solution of in alphairon. It is having BCCcrystal structure.It is relativity soft,ductile and strongly magnetic. Austenite Austenite is a sold solution of carbon in gama-iron.It is having BCC crystal structure. It is generally soft and ductile than ferrite ,but denser than ferrite ,and is non-megnetic. Cementite Cementite is a chemical compound of 93.33% and 6.67% carbon.It is having orthorhombic structure. It is having round particals in the strycture.It is extremely hard having about1400 brinell and brittle. It has no ductility. Pearlite: Pearlite can be recognized by its pearly lustruous appearance and its structure of thin alternating plates of 13 per cent Pearlite is a strong metalhaving medium hardness. 1 2 3 4 Crystal Structure cementite in a matrix of 87 per cent ferrite. Ledeburite 5 Sorbite: 6 7 Ledeburite mixture of austenite and cementite. It is reeated hard. Sorbite it is a finely dispersed perlite, and the properties are intermediate between those of pearlite and troostite. It is related hard. Troostite Troostite or bainite or It is the most finely baninite acicular (needlelike) structure of austenite decomposition. It is composed of two equilibrium phases of ferrite and cementite. It has mediunm Hardness. 8 Martensite Martensite is obtanined by decomposition of austenite when it is very apidly cooled . It is composed or needle-like crystal in angular arrangements Martensite is very hard, brittle and magnetic. COMMON ENGINEERING MATERIAL FOR WORK AND TOOLS The common material used in the workshop for the work and tools can be grouped in two category as metallic or non-metallic. The metallic material are further classified as ferrious and Non-ferrous mater. The ferrous material consists of mainly Ironwith comparitelively small addition of other elements. Non Ferrous material contains or no iron. FERROUS METAL Ferrous metal are Iron and its alloy such as pig iron , cast iron , wrought iron and steels. PIG IRON On processing iron in the blast furnace pig iron is obtained. Pig iron contains about 92% iron and other element like carbon silicon, magnese, sulphur and phosphorous. Pig iron is used to get cast iron and wrought by remelting. CAST IRON Cast iron is an alloy of iron , carbon and silicon . The carbon content in the cast iron varies from 1%to 3% is obtained by reelting and refining the pig in a cupola furnace. The presence of carbon in the caast iro is either in the form of the free carbon (grapite) or in the form of iron carbide.Fe3C. GREY CAST IRON Grey cast iron contents crbon in the form form of or in the form of the graphite . It is known grey cast iron on account of itsgreyish structure . It is having high compressive strength and damping property . The material have excellence machiibility due to presence of free carbon and a low cost. It is widely been used for making components for machine used for supporting the other part of the assembley such as base, etc. It can be shaped continently by casting process. Cast iron is a brittle material lacking in ducktility and poor in shocks resistanc. It has restricted yse for components subjected to tensile stress and sudden applied loads. WHITE CAST IRON In white cast iron carbon is present in the form of iron carbide. It has whitish appearance. Iron carbide is a hard and brittle substance and presence increase the hardness in the casting. White cast iron is almost unmachinable and has got the restricted use for the parts . which required abrasion resistance. For processing malleable cast iron castings are made from white cast ironand then it is treated to convert them in to malleable cast iron casting. WROUGHT IRON Wrought iron is a highly refined iron with small amount ofslag forged out in to fibre. It can have purity of 99.9% iron and the impurities of silicates may be in the form of mechanical mixture. It has important properties of ductility and malleability and toughness. Wrought iron is suitable for machine parts to shaped forging . It has excellent weldability. Bolts and nuts, chains,crains hooks railway couplings , pipes and pipe fitting , plates, bar and boiler are theprinciple form in which Wrought iron is used. STEEL It is an alloy of carbon in which carbon content is less than 10.5% .It also contains the small% of phosphorous ,magnese etc. but the carbon , is most important modifying element. Steels are classified as: 1. Plain carbon steel 2. Speccial carbon steel 3. Alloy steel Plain carbon steel according their carbon contents may be divided in to 4 division as: Low carbon or mild steel 0.05 to 0.45% carbon Medium carbon steel 0.45 to 0.80%carbon High carbon steel 0.80 to 1.65 % carbon Tool steel 1.00 to 1.50% carbon The mild steel and the medium carbon steel are widely used, as basic raw material for many machine components, Whle high carbon and tool steel mainly used for making tools. MILD STEEL The low carbon steel has been sub divided as dead mild steel and mild steel . Dead mile steel contains carbon up to 0.1%. It is softest and most ductile metal and has excellent machinibility and weldebility . It can be rolled in to sheets . It is also used for rivets and solid drawn steel tubes. Mild steel contains carbin ranging from 0.1% to 0.45%. It has excellent welding properties and machinibility .It is harder than dead mild steel but less ductile . It is mainly used for plate work, bar for generalwork and rolled steel for section for structural work. MEDIUM CARBON SEEL Medium carbon steel contains carbon ranging from 0.45% to 0.8%. With increasing carbon content the strength end hardness increase but ductility decreases .Increasing carbon content also affecting the welding properties and machinability. Axies,gears,shafts, rals are some of the well- known components of medium carbon steel on account of high strength and wear resistance . The material has recolling properties as such also used for making spring. HIGH CARBON AND TOOL STEEL High carbon and tool steel contains rangingfrom0.45 to 0.8 %.With increasing content the strength and hardness increases but ductility decreases, Incresing carbon content also affecting the welding properties and machinibility. Axess, gears ,shaft, rails are some of the well-known components of medium carbon steel on account of high strength and wear resistance. The material has recolling properties as such also used for making springs. HIGH SPEED STEEL High –speed steel is an alloy in which element other than carbon is added in sufficient amount to achievebetter properties. High heat hardness and wear resistance restrict the use of plain carbon steel , to cut metal at low cutting speed. Alloying is done for specific purpose to improve high heat hardness, wear resistance, corrosion resistance, electric and magnetic properties . Nickel, chromium, molybdenium, cobalt, vanadium, magnese, silicon and cobalt are some of the important alloying elements. NICKEL Nickel in steel contribute great strength and hardnes. With high elastic limit and good ductility and good resistance to corrosuion. CHROMIUM The present of chromium improves hardness combined with high strength and high elastic limit . It also improve strength. The large% of chromium in stainless steel is responsible for corrosion resistance. TUNGSTON It improves the hot harness , which makes possible to cut at high cutting speed. Vanadium Vanadium along with chromium has extremely good tensile strength,elasticlimit and ductility.These steels are frequently used for parts such as spring, shaft gear etc. MANGANESE Manganese improves the strength of steel in both hot rolled and heat-treated condition SILICON Silicon steel hass the properties similar to nickel steel. COBALT It is added to the steel to improve the hot hardness. High-speed steel is the general purpose metal for low and medium cutting speeds owing to its superiors hot-hardness and resistance to wear 18:4:1 high speed contains 18%tungston4% chromium and 1%vanadium is considered as an important all purpose cutting tool material. COST COMPARISION CHART Iron-carbon phase diagram Iron-carbon phase diagram describes the iron-carbon system of alloys containing up to 6.67% of carbon, discloses the phases compositions and their transformations occurring with the alloys during their cooling or heating. Carbon content 6.67% corresponds to the fixed composition of the iron carbide Fe3C. The diagram is presented in the picture: The following phases are involved in the transformation, occurring with iron-carbon alloys: L - Liquid solution of carbon in iron; δ-ferrite – Solid solution of carbon in iron. Maximum concentration of carbon in δ-ferrite is 0.09% at 2719 ºF (1493ºC) – temperature of the peritectic transformation. The crystal structure of δ-ferrite is BCC (cubic body centered). Austenite – interstitial solid solution of carbon in γ-iron. Austenite has FCC (cubic face centered) crystal structure, permitting high solubility of carbon – up to 2.06% at 2097 ºF (1147 ºC). Austenite does not exist below 1333 ºF (723ºC) and maximum carbon concentration at this temperature is 0.83%. α-ferrite – solid solution of carbon in α-iron. α-ferrite has BCC crystal structure and low solubility of carbon – up to 0.25% at 1333 ºF (723ºC). α-ferrite exists at room temperature. Cementite – iron carbide, intermetallic compound, having fixed composition Fe3C. Cementite is a hard and brittle substance, influencing on the properties of steels and cast irons. The following phase transformations occur with iron-carbon alloys: Alloys, containing up to 0.51% of carbon, start solidification with formation of crystals of δferrite. Carbon content in δ-ferrite increases up to 0.09% in course solidification, and at 2719 ºF (1493ºC) remaining liquid phase and δ-ferrite perform peritectic transformation, resulting in formation of austenite. Alloys, containing carbon more than 0.51%, but less than 2.06%, form primary austenite crystals in the beginning of solidification and when the temperature reaches the curve ACM primary cementite stars to form. Iron-carbon alloys, containing up to 2.06% of carbon, are called steels. Alloys, containing from 2.06 to 6.67% of carbon, experience eutectic transformation at 2097 ºF (1147 ºC). The eutectic concentration of carbon is 4.3%. In practice only hypoeutectic alloys are used. These alloys (carbon content from 2.06% to 4.3%) are called cast irons. When temperature of an alloy from this range reaches 2097 ºF (1147 ºC), it contains primary austenite crystals and some amount of the liquid phase. The latter decomposes by eutectic mechanism to a fine mixture of austenite and cementite, called ledeburite. All iron-carbon alloys (steels and cast irons) experience eutectoid transformation at 1333 ºF (723ºC). The eutectoid concentration of carbon is 0.83%. When the temperature of an alloy reaches 1333 ºF (733ºC), austenite transforms to pearlite (fine ferrite-cementite structure, forming as a result of decomposition of austenite at slow cooling conditions). Critical temperatures Upper critical temperature (point) A3 is the temperature, below which ferrite starts to form as a result of ejection from austenite in the hypoeutectoid alloys. Upper critical temperature (point) ACM is the temperature, below which cementite starts to form as a result of ejection from austenite in the hypereutectoid alloys. Lower critical temperature (point) A1 is the temperature of the austenite-to-pearlite eutectoid transformation. Below this temperature austenite does not exist. Magnetic transformation temperature A2 is the temperature below which α-ferrite is ferromagnetic. Phase compositions of the iron-carbon alloys at room temperature Hypoeutectoid steels (carbon content from 0 to 0.83%) consist of primary (proeutectoid) ferrite (according to the curve A3) and pearlite. Eutectoid steel (carbon content 0.83%) entirely consists of pearlite. Hypereutectoid steels (carbon content from 0.83 to 2.06%) consist of primary (proeutectoid)cementite (according to the curve ACM) and pearlite. Cast irons (carbon content from 2.06% to 4.3%) consist of proeutectoid cementite C2 ejected from austenite according to the curve ACM , pearlite and transformed ledeburite (ledeburite in which austenite transformed to pearlite).