Powerpoint
advertisement

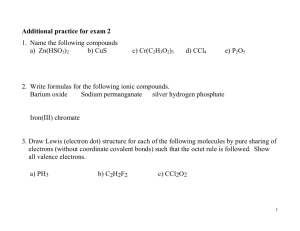
Metals tend to have one, two or three electrons in their outer orbits and they tend to lose these electrons when they combine with other elements to form compounds. Non-metals tend to have five, six or seven electrons in their outer orbits so they gain electrons when they combine with metals to form compounds. IONIC COMPOUNDS – When atoms lose or gain electrons to become stable, they form cations or anions. Cations and anions are oppositely charged and are attracted to each other – this explains why some compounds are formed. These compounds are relatively harmless compounds because the elements in the compound are stable. These compounds are called ionic compounds which are compounds made up of positive and negative ions that have resulted from the transfer of electrons from a metal to a nonmetal. Sodium chloride (table salt) is an ionic compound made up of sodium ions, Na+, and chloride ions, Cl-. For ionic compounds that contain only two elements, one element is always a metal and the other is a non-metal. The attraction that holds oppositely charged ions together in a compound is called an ionic bond. Aluminum and Chlorine gas combine to form an ionic compound called aluminum chloride. Each aluminum atom has three electrons to lose, while each chlorine atom can accommodate only one extra electron. This means that aluminum reacts with three chlorine atoms. Draw a diagram to show the transfer of electrons from calcium to fluorine. Draw a diagram to show the transfer of electrons from calcium to fluorine. F 1- F 1Ca2+ Calcium and Fluorine combine to form calcium fluoride, an ionic compound. Each calcium atom has 2 electrons in its valence shell so it wants to lose both to become stable. Fluorine has 7 electrons in its valence shell so it wants to gain one electron to become stable. Therefore, one Calcium atom needs two fluorine atoms to form compound Calcium Fluoride. Ionic compounds are compounds made up of one metal and one non-metal. The elements by themselves have ionic charges but they come together to make a neutral compound (positive charge = negative charge). Naming Ionic Compounds Names of ionic compounds have two parts. The first part refers to the metal ion in the compound and the second part to the non-metal ion. Remember that the name of the metal ion remains the same as the metal atom but the ending of the name of the second ion (non-metal) changes to “ide”. Group 1 = 1 valence electron - forms 1+ ions Group 2 = 2 valence electrons - forms 2+ ions Group 13 = 3 valence electrons - forms 3+ ions Group 14 = 4 valence electrons - forms 4+ ions But Group 14 is special since it is just as easy to add 4 electrons and achieve a full valence shell (depending on certain conditions). So, Group 14 = Group 15 = Group 16 = Group 17 = Group 18 = 4 valence electrons- forms 4- ions 5 valence electrons- forms 3- ions 6 valence electrons- forms 2- ions 7 valence electrons- forms 1- ions 8 valence electrons - form NO IONS!! We can use these valences values in writing chemical formulas. Writing Chemical Formulas of Ionic Compounds Valence is important in writing the chemical formulas of ionic compounds. The simplest way to tell what the valence value of an element will be is to look at the periodic table. Writing Chemical Formulas of Ionic Compounds “The Zero-Sum Rule” – The sum of all charges in the chemical formula of the compound must equal zero. Example 1: Write the chemical formula of magnesium chloride. Step 1: Write the symbols of the elements, with the metal on the left side and the non-metal on the right side. Mg Step 2: Cl Add the ionic charge of each ion above the symbol. +2 Mg -1 Cl Step 3: Determine how many ions of each type are required to bring the total charge to zero. The sum of all charges in the compound must equal zero. Total charge: Step 4: 1(+2) + 2(-1) = 0 Mg Cl Write the chemical formula using the (bold) coefficients in front of each bracket as subscripts. Mg1Cl2 Step 5: Do not write the subscript “1” in chemical formulas because the symbol itself represents on ion. The chemical formula for magnesium chloride is MgCl2. Example 2: Write the chemical formula of aluminum oxide. Step 1: Write the symbols of the metal and non-metal elements. Al Step 2: Add the ionic charge of each ion above the symbol. +3 Al Step 3: -2 O Determine the number of ions required to bring the total charge to zero. 2(+3) Al Step 4: O 3(-2) = 0 O The chemical formula of aluminum oxide is Al2O3. The Crisscross Method for Determining Formulas Step 1: Write out the chemical symbols for each elements involved in the ionic compound. Write the metal (cation) first, followed by the non-metal (anion). Magnesium Mg Step 2: Chlorine Cl Write the ionic charge (valence) above each symbol based on which group they fall under in the periodic table. Mg 2+ 1 Cl 12 Notice that there are no longer Step 3: Crossover the numbers, drop the signs, and write them as subscripts. We do not show the 1 because the symbol itself Mg represents one element. We no longer show a + or – because when the charged ions bond together their respective valence shell is full and the compound becomes neutral. MgCl2 Step 4: Look for the common factor between both subscripts. It one exists, decrease each subscript by this factor. If not, leave the formula. MgCl2 (No common factor exists here) Step 5: Name the compound by changing the ending of the non- metal element from –ine / -gen to –ide. MgCl2 = magnesium chloride Elements with Multiple Ionic Charges We have a special set of elements that are multivalent (multi = many, valent = valences). They are located in the Transition Metals (centre of the periodic table). Examples are: Copper Iron Lead Nickel Manganese Tin Gold Example 1: Write the chemical name of CuBr2. Step 1: Since we do not know whether the copper ion is +1 or +2, we reverse our criss-cross to see what the charge will be. 2 Cu 1 1 Br Reverse the criss-cross 2 Therefore, the ionic charge of copper in this compound is +2. Step 2: Write the name of the compound. When the metal has more than one ionic charge you must always indicate the ionic charge with Roman numerals. The name of CuBr2 is copper (II) bromide. The following are some other examples: Copper can have multiple ionic charges. What are the possibilities? You can find these charges in your periodic table. Copper can have either a charge of 1+ or 2+. So for example, when copper forms a compound with chlorine the chemical name and chemical formula will be the following: Copper (I) chloride (CuCl) Copper (II) chloride (CuCl2) Iron can have multiple ionic charges. What are the possibilities? Iron can have a charge of 2+ or 3+. So for example, when iron forms a compound with oxygen the chemical name and chemical formula will be the following: Iron (II) oxide (FeO) Iron (III) oxide (Fe2O3)