Hints for Empirical Formula Lab
advertisement

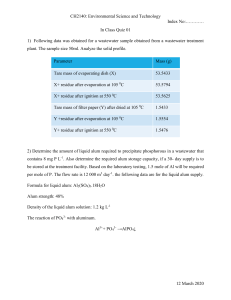
Analysis of Alum Lab: • Don’t heat too strongly at first, to avoid too much hydrate bubbling out of the crucible • ALL Steps — ALWAYS use crucible tongs (no fingers). Analysis of Alum Lab: • Scales do not measure accurately when they are not at room temperature (make sure everything is cool before placing on scale). • Make sure scales are measuring grams (not ounces) and set to zero before measuring Analysis of Alum Lab: • • • Always test the hot crucible/lid before assuming it is cool (don’t get burned!) Keep lab goggles on at ALL TIMES. Clean up station completely; wash hands. Analysis : Show all calculations for #1, 3; Write complete sentences for #2, 3, 4, 5 Conclusion: --short statement of the final results from the lab (what was the complete formula for alum, what was your % error) --short statement about the significance of the lab (why it is important for a chemistry student to conduct this lab)