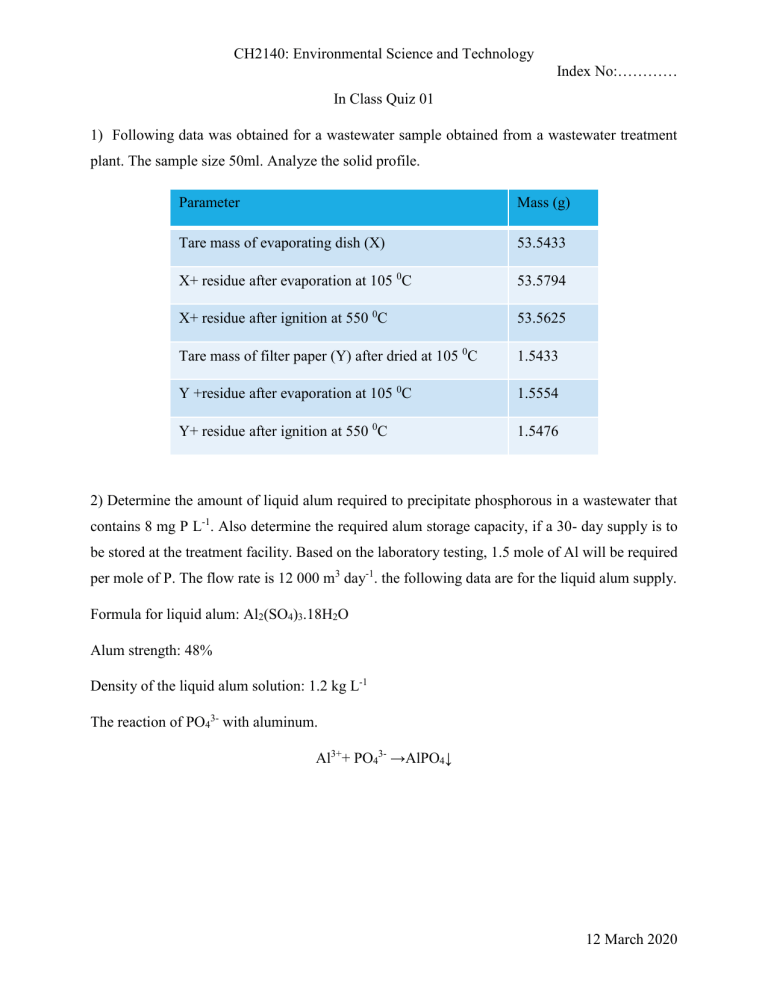
CH2140: Environmental Science and Technology Index No:………… In Class Quiz 01 1) Following data was obtained for a wastewater sample obtained from a wastewater treatment plant. The sample size 50ml. Analyze the solid profile. Parameter Mass (g) Tare mass of evaporating dish (X) 53.5433 X+ residue after evaporation at 105 0C 53.5794 X+ residue after ignition at 550 0C 53.5625 Tare mass of filter paper (Y) after dried at 105 0C 1.5433 Y +residue after evaporation at 105 0C 1.5554 Y+ residue after ignition at 550 0C 1.5476 2) Determine the amount of liquid alum required to precipitate phosphorous in a wastewater that contains 8 mg P L-1. Also determine the required alum storage capacity, if a 30- day supply is to be stored at the treatment facility. Based on the laboratory testing, 1.5 mole of Al will be required per mole of P. The flow rate is 12 000 m3 day-1. the following data are for the liquid alum supply. Formula for liquid alum: Al2(SO4)3.18H2O Alum strength: 48% Density of the liquid alum solution: 1.2 kg L-1 The reaction of PO43- with aluminum. Al3++ PO43- →AlPO4↓ 12 March 2020






