Chemical Reactivity Hazards Management
advertisement

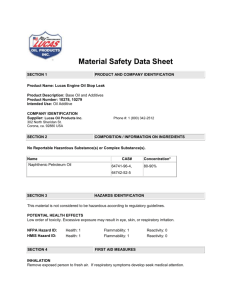
Introduction to Chemical Reactivity Hazard Management Mikal Shabazz U.S. Environmental Protection Agency Mid-Atlantic Region, Office of Enforcement Oil and Prevention Branch Overview What is Chemical Reactivity? What are its Hazards? Challenges in Managing Reactive Hazards Examples of Facilities with Reactive Chemistry Hazards Screening Example using Case History Principles of Reactive Hazard Management Tools and Resources 2 3 Uses of Reactive Chemistry 5 6 7 What energy/products will be released? • Initial conditions • Reaction path • Reaction thermodynamics • Reaction kinetics AND How will the released energy/products interact with the environment? • Environmental conditions • Process equipment & controls • Heat & mass transfer • People/property/environment response 9 Types of Chemical Reactivity Main Category Subcategory Readily SelfReacts Polymerizes Decomposes Rearranges Condenses Readily Reacts with Common Environmental Substances Reacts with Nitrogen Reacts with Oxygen Reacts with Water Reacts with Ordinary Combustibles Reacts with Metals Readily Reacts with Other Chemicals 10 Reacts with Acids Reacts with Bases Reacts with Hydrogen The Problem Chemical Safety Board Report • 167 incidents in a 21 year period • 108 fatalities • Significant property damage 11 Data are incomplete, and certainly underestimate the magnitude of the problem The Problem 12 In 90% + of all incidents studied, the information necessary to have prevented the incident was documented and publicly available. Groups that Use Reactive Chemistry “No Chemistry” Users “Unintentional Chemistry” Users “Intentional Chemistry” Users Or, stated another way Storage, Handling and Repackaging Mixing and Physical Processing Chemical Manufacturing 13 Preliminary Screening Method Management System Framework Concept Book(~ 200 pages) FREE DOWNLOAD http://www.aiche.org/ccps/resources.htm 14 Guiding Principles 1. Use existing information 2. Apply appropriate levels of technology to the level of the problem 3. Identify areas requiring additional testing, data generation 4. Use existing management systems/ structures to the maximum extent possible 15 Recommended Approach Preliminary Screening Method Management System Framework 16 Preliminary Screening for Chemical Reactivity Hazards Summary Flowchart Preliminary Screening: Summary Flowchart Summary Flowchart (continued) FACILITY: COMPLETION DATE: COMPLETED BY: APPROVED BY: Do the answers to the following questions indicate chemical reactivity hazard(s) are present? _____________ YES, NO or NA AT THIS FACILITY: Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical processing of substances? 7. Is any hazardous material identified as spontaneously combustible? 8. Is any hazardous material identified as peroxide forming? 9. Is any hazardous material identified as water reactive? 10. Is any hazardous material identified as an oxidizer? 11. Is any hazardous material identified as self-reactive? 12. Can incompatible materials coming into contact cause hazardous consequences, based on the following analysis? BASIS FOR ANSWER; COMMENTS Question 1. SCENARIO CONDITIONS * NORMAL? Example Form for Documenting Chemical Reactivity Hazard Screening R, NR ** or ? INFORMATION SOURCES; COMMENTS 1 2 3 *Does the contact/mixing occur at ambient temperature, atmospheric pressure, 21% oxygen atmosphere, and unconfined? (IF NOT, DO NOT ASSUME THAT PUBLISHED DATA FOR AMBIENT CONDITIONS APPLY) **R = Reactive (incompatible) under the stated scenario and conditions NR = Non-reactive (compatible) under the stated scenario and conditions ? = Unknown; assume incompatible until further information is obtained An Example Simplified retrospective of 1995 explosion / fire at Napp Technologies, Lodi, New Jersey Intent: Illustrate the Preliminary Screening Method for the type of process involved in the incident 21 Mixing Example A toll manufacturer is contracted to prepare one 8100 lb batch of a gold precipitating agent. • Ingredients mixed in a 125 ft3 (6 m3) cone blender • Blender is insulated and has a steel jacket to allow cooling and heating with a water/glycol mixture • Dry ingredients blended: ~ 66% sodium hydrosulfite, 22% aluminum powder and 11% potassium carbonate by weight • Small amount of benzaldehyde added for odor control • Product blend packaged into eighteen 55 gal drums for shipment 22 FACILITY: COMPLETED BY: Mixing Example Documentation APPROVED BY: COMPLETION DATE: Do the answers to the following questions indicate chemical reactivity hazard(s) are present? _____________ AT THIS FACILITY: YES, NO or NA BASIS FOR ANSWER; COMMENTS R, NR ** or ? INFORMATION SOURCES; COMMENTS Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical processing of substances? 7. Is any hazardous material identified as spontaneously combustible? 8. Is any hazardous material identified as peroxide forming? 9. Is any hazardous material identified as water reactive? 10. Is any hazardous material identified as an oxidizer? 11. Is any hazardous material identified as self-reactive? 12. Can incompatible materials coming into contact cause hazardous consequences, based on the following analysis? Question 1. SCENARIO 1 2 3 CONDITIONS * NORMAL? Sources of Information Here are some key sources of reactivity information, if you know what chemicals are being handled. • International Chemical Safety Cards - accessible from CDC website at http://www.cdc.gov/niosh/ipcs/icstart.html) • NIOSH Pocket Guide to Chemical Hazards - online version available at http://www.cdc.gov/niosh/npg/npg.html) • NFPA Fire Protection Guide to Hazardous Materials [NFPA 2002] • Bretherick's Handbook of Reactive Chemical Hazards [Urben 1999] • Coast Guard Hazardous Information System http://www.chrismanual.com • NOAA Chemical Reactivity Worksheet - discussed later in this session - http://response.restoration.noaa.gov/chemaids/react • Sax's Dangerous Properties of Industrial Materials [Lewis and Irving 2000] • Data from manufacturers/suppliers, including Material Safety Data Sheets (MSDSs) 24 Material Safety Data Sheets Basic reactivity hazards of each chemical used should be included the Material Safety Data Sheet provided by your chemical supplier. In the U.S., the OSHA HAZCOM Standard says what must be included in an MSDS, but does not give a required format. 25 Material Safety Data Sheets The widely used ANSI Standard Z400.1 gives a consistent format for MSDSs. Reactivity information might be found in: • Section 5: Fire-Fighting Measures Look for water reactivity and the consequences of heating the material in a fire situation, including decomposition, polymerization, and the generation of gaseous reaction products. • Section 10: Stability and Reactivity The primary section for reactivity hazards. Look for information on chemical stability, conditions to avoid, incompatibility with other materials, hazardous decomposition, and hazardous polymerization. • Section 14: Transport Information Look for the hazardous material description, the hazard class, and the UN/NA identification number. One place to look up what the classes and numbers mean is in the DOT Emergency Response Guidebook. • Section 16: Other Information Look for hazard ratings (such as the NFPA instability rating) and other possible clues such as how the material is prepared. 26 Material Safety Data Sheets WARNING: • MSDSs often contain incomplete or contradictory information. The U.S. EPA issued a Safety Alert warning not to rely on a single data source in emergency situations. • The same is true when attempting to identify reactivity hazards that need to be avoided or managed. Other MSDS limitations are listed in Section 4.2 of the CCPS Essential Practices book. 27 Screening Question 1 Question 1 Is intentional chemistry performed at your facility? Mixing Example Documentation AT THIS FACILITY: Question 1. Is intentional chemistry performed? YES, NO or NA NO BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Question 2 Is there any mixing or combining of different substances? Mixing Example Documentation AT THIS FACILITY: Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? YES, NO or NA NO YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender Question 6 Is any heat generated during the mixing or physical processing of substances? Mixing Example Documentation AT THIS FACILITY: Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? YES, NO or NA NO YES NA NA NA BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender Mixing Example Documentation AT THIS FACILITY: Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical processing of substances? YES, NO or NA NO YES NA NA NA NO BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Question 7 Is any substance identified as spontaneously combustible? Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating Expect chemical reactivity hazard(s) to be present; Go to Chapter 4 for information on identifying and managing hazards Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES 8. Is any substance identified as peroxide forming? 9. Is any substance identified as water reactive? 10. Is any substance identified as an oxidizer? 11. Is any substance identified as self-reactive? 12. Can incompatible materials coming into contact cause undesired consequences, based on the following analysis? YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES 8. Is any substance identified as peroxide forming? 9. Is any substance identified as water reactive? 10. Is any substance identified as an oxidizer? 11. Is any substance identified as self-reactive? 12. Can incompatible materials coming into contact cause undesired consequences, based on the following analysis? NO YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating No indication from MSDS or literature Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES 8. Is any substance identified as peroxide forming? 9. Is any substance identified as water reactive? NO YES 10. Is any substance identified as an oxidizer? 11. Is any substance identified as self-reactive? 12. Can incompatible materials coming into contact cause undesired consequences, based on the following analysis? YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating No indication from MSDS or literature Sodium hydrosulfite is water reactive; uncoated aluminum powder is DOT/UN Hazard Class 4.3, Dangerous when Wet Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES 8. Is any substance identified as peroxide forming? 9. Is any substance identified as water reactive? NO YES 10. Is any substance identified as an oxidizer? 11. Is any substance identified as self-reactive? NO 12. Can incompatible materials coming into contact cause undesired consequences, based on the following analysis? YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating No indication from MSDS or literature Sodium hydrosulfite is water reactive; uncoated aluminum powder is DOT/UN Hazard Class 4.3, Dangerous when Wet No indication from MSDS or literature Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES 8. Is any substance identified as peroxide forming? 9. Is any substance identified as water reactive? NO YES 10. Is any substance identified as an oxidizer? 11. Is any substance identified as self-reactive? NO YES 12. Can incompatible materials coming into contact cause undesired consequences, based on the following analysis? YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating No indication from MSDS or literature Sodium hydrosulfite is water reactive; uncoated aluminum powder is DOT/UN Hazard Class 4.3, Dangerous when Wet No indication from MSDS or literature Heating of sodium hydrosulfite can initiate self-sustaining exothermic decomposition Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES 8. Is any substance identified as peroxide peroxideforming? forming? 9. Is any substance identified as water water reactive? reactive? NO YES 10. Is any substance identified as an oxidizer? oxidizer? 11. Is any substance identified as self-reactive? self-reactive? NO YES 12. Can incompatible materials coming into contact cause undesired consequences, based on the following analysis? YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating No indication from MSDS or literature Sodium hydrosulfite is water reactive; uncoated aluminum powder is DOT/UN Hazard Class 4.3, Dangerous when Wet No indication from MSDS or literature Heating of sodium hydrosulfite can initiate self-sustaining exothermic decomposition Mixing Example Documentation Do the answers to the following questions indicate chemical reactivity hazard(s) are present? AT THIS FACILITY: YES, NO or NA Question 1. Is intentional chemistry performed? 2. Is there any mixing or combining of different substances? 3. Does any other physical processing of substances occur? 4. Are there any hazardous substances stored or handled? 5. Is combustion with air the only chemistry intended? 6. Is any heat generated during the mixing or physical NO YES NA NA NA NO processing of substances? 7. Is any substance identified as spontaneously combustible? YES 8. Is any substance identified as peroxide forming? 9. Is any substance identified as water reactive? NO YES 10. Is any substance identified as an oxidizer? 11. Is any substance identified as self-reactive? NO YES 12. Can incompatible incompatiblematerials materials coming coming intointo contact contact cause undesired cause undesired consequences, consequences? based on the following analysis? YES BASIS FOR ANSWER; COMMENTS Loading, blending, and packaging only Blending of ingredients in cone blender No indication of heat generation from previous batch or from nature of blend Sodium hydrosulfite is DOT/UN Hazard Class 4.2, Spontaneously Combustible Material; finely divided aluminum powder is pyrophoric without oxide coating No indication from MSDS or literature Sodium hydrosulfite is water reactive; uncoated aluminum powder is DOT/UN Hazard Class 4.3, Dangerous when Wet No indication from MSDS or literature Heating of sodium hydrosulfite can initiate self-sustaining exothermic decomposition Chemical Compatibility Determinations Public literature (e.g., Sax, Brethericks, MSDS, etc.) Chemical Compatibility Charts (ASTM E 2012-00 – Standard Guide for the Preparation of a Binary Chemical Compatibility Chart, Coast Guard chart, etc.) NOAA Chemical Reactivity Worksheet 46 NOAA Chemical Reactivity Worksheet The Chemical Reactivity Worksheet is a PC Application written by the National Oceanic and Atmospheric Administration (NOAA). It is included on the CD available at this session, or as a free download from the NOAA website. The page which contains the download link as well as other information on the worksheet is: http://response.restoration.noaa.gov/chemaids/react.html 47 NOAA Chemical Information 48 NOAA Compatibility Chart 49 NOAA Compatibility Chart 50 FACILITY: Mixing Example Documentation Napp Technologies, Inc., Lodi, New Jersey (continued) Do the answers to the following questions indicate chemical reactivity hazard(s) are present? 1 ___YES____ AT THIS FACILITY: Question 1. Is intentional chemistry performed? 12. Can incompatible materials coming into contact cause 2. Is thereconsequences, any mixing or combining of different undesired based on the followingsubstances? analysis? 3. Does any other physical processing of substances occur? CONDITIONS SCENARIO 2 4. Are there any hazardous substances stored or NORMAL? handled? 1 Vacuum seal cooling water enters blender,intended? No – N2 5. Is combustion with air the only chemistry powder atmosphere, 6. reacts Is anywith heataluminum generated duringand the sodium mixing or physical hydrosulfite, and initiates exothermic confinement processing of substances? decomposition in blender 7. Is any substance identified as spontaneously combustible? 2 Glycol/water mixture leaks from jacket into No – N2 blender, reacts with aluminum powder and atmosphere, sodium hydrosulfite, and initiates confinement decomposition in blender 8. exothermic Is any substance identified as peroxide forming? YES, NO or NA NO YES YES R,NA NR 3 orNA ? R NA NO BASIS FOR ANSWER; COMMENTS Loading, blending, See analysis belowand packaging only analysis below Blending of ingredients in cone blender INFORMATION SOURCES; COMMENTS Both aluminum powder and sodium hydrosulfite reactive from No indicationare of water heat generation previous batch or from nature of blend YES Sodium hydrosulfite is DOT/UN Hazard R NOAA4.2, Worksheet indicatesCombustible combining Class Spontaneously sodium hydrosulfite withaluminum ethylenepowder glycol Material; finely divided is pyrophoric “explosive due to vigorous reaction or is without oxide coating reaction products produce NO No indication frommay MSDS or literature detonation,” “may cause fire,” and Sodium water reactive; 9. Is any substance identified as water reactive? YES indicateshydrosulfite “flammableisgas generation” uncoated is DOT/UN and “heat aluminum generationpowder by chemical Hazard Dangerous When Wet reaction,Class may 4.3, cause pressurization” 10. Is any substance identified as an oxidizer? NO No indication from MSDS or literature 1 Use Figure 3.1 with answers to Questions 1-12 to determine if answer is YES or NO 11. Is any substance identified as self-reactive? YES Heating of sodium hydrosulfite can 2 Does the contact/mixing occur at ambient temperature, atmospheric pressure, 21% oxygen atmosphere, and initiate self-sustaining exothermic unconfined? (IF NOT, DO NOT ASSUME THAT PUBLISHED DATA FOR AMBIENT CONDITIONS APPLY) 3 decomposition R = Reactive (incompatible) under the stated scenario and conditions NR =Can Non-reactive (compatible) stated scenario 12. incompatible materials under comingtheinto contact causeand conditions YES See analysis below ? = Unknown; assume incompatible until further information is obtained undesired consequences, based on the following analysis? An Example For this retrospective example: Preliminary Screening Method would indicate that chemical reactivity hazards need to be managed at this facility Multiple indicators present • Individual chemicals are spontaneously combustible, water reactive, thermally sensitive • Interaction scenarios indicate potential incompatibilities 52 Preliminary Screening for Chemical Reactivity Hazards Summary Flowchart Incident April 21, 1995 5 worker fatalities ~300 evacuated Facility destroyed Surrounding businesses damaged Ed Hill, The Bergen Record Used with permission 54 Appropriate Management Systems Identification of the reactive nature and interaction of the materials Use of a compatible heating / cooling medium Mechanical integrity program to decrease the likelihood of leaks Training of personnel (operations and emergency response) on possible upset situations and appropriate response 55 Essential Management Practices 4.1 Put Into Place a System to Manage Chemical Reactivity Hazards 4.2 Collect Reactivity Hazard Information 4.3 Identify Chemical Reactivity Hazards 4.4 Test For Chemical Reactivity 4.5 Assess Chemical Reactivity Risks 4.6 Identify Process Controls and Risk Management Options 56 Essential Management Practices 4.7 Document Chemical Reactivity Risks and Management Decisions 4.8 Communicate and Train on Chemical Reactivity Hazards 4.9 Investigate Chemical Reactivity Incidents 4.10 Review, Audit, Manage Change and Improve Hazard Management Practices and Program 57 Summary Preliminary screening method • Useful for identifying where chemical reactivity hazards are likely to exist Management system framework • Applicable to all levels of complexity and sophistication • Builds on existing management systems • Supplemented with tools appropriate for chemical reactivity hazards 58 Ongoing Reactivity Initiatives OSHA Chemical Reactivity Hazards Management Alliance • http://www.osha.gov/SLTC/reactivechemicals/ Reactivity Management Roundtable • http://www.aiche.org/CCPS/ActiveProjects/RMR/ index.aspx 59 Reactive Hazard Resources CD The CD available at this session includes the following: • Complete text of CCPS concept book • CSB reactive hazards investigation reports and alerts • NOAA Reactivity Worksheet • Collection of reactive chemical MSDSs • EPA chemical reactivity safety alerts • This presentation 60