Photosynthesis
advertisement

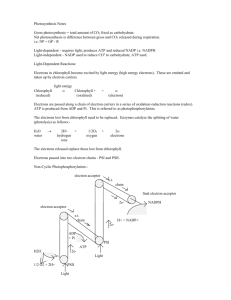
The “Photo” in Photosynthesis To understand the major thrust of photosynthesis, ask yourself this question: what does it take for a plant to synthesize a carbohydrate? If you said it takes energy and a carbon source, then you know the basic outline of photosynthesis. Energy comes from the sun in the form of photons, carbon as CO2 from the air. Now, ask yourself “what remarkable system must a plant have to harness radiant energy of a photon? And, “what remarkable system must the plant have to trap CO2 and use this as a carbon source to make glucose, sucrose and starch? This tutorial answers the first question. How should you picture the energy in light? Physicists tell us that light is both a wave and a particle that is manifested as electromagnetic and electric fields. Whatever that means, remember that light energy depends on its frequency, which is defined as the number of wave length units that can be fitted into a space of one second. The more units, the greater the frequency (click 1). Light energy is measured in units called photons. The amount of energy in a photon varies, but not in a continuous manner. Rather photon energy occurs in steps or packets called quanata (click 1). The steps are defined by the equation that relates energy to frequency of light (click 1). The h in the equation is Planck’s constant and has a value of 6.626 x 10-34 Joules x sec. The Greek symbol v or “nu” refers to frequency. E = h In the illustration on the right, the upper curve has a shorter wave length (measured as red) than the lower curve. Thus, based on the formula, one could say that the upper curve has twice the energy of the lower curve. More importantly, a photon of light with a greater frequency can impart more energy to the plant. But, the plant must be receptive to the energy. Click 1 to go on. 2 per second 1 per second One second Chlorophyll, a green pigment, allows plants to absorb light energy. Energy absorption, however, must be consistent with allowable (basal to excited state) electron transitions within the chlorophyll molecule (click 1). Because these transitions are not continuous, a plant obtains energy only at certain frequencies of light. Energy insufficient to reach an excited state is not absorbed (click 1). Similarly, energy that drives an electron past one energy level but is insufficient to reach a second is not absorbed (click 1). To be absorbed, the energy must be sufficient to reach only allowable energy states (click 1). This simple rule of quantum physics is all you need to know to understand an absorption spectra of chlorophyll (click 1). 2 Chlorophyll b Absorption Intensity excited states 1 ground state Chlorophyll a 300 400 500 600 700 Higher plants have two photocenters, P680 and P700, so designated by the wavelength that gives maximum absorption or O2 evolution. Assume you wish to design a photosystem in a plant. You need a membrane-bound enclosure with a hollow center (click 1). Next, you need a photocenter that serves as an electron source (click 1). To replace the departing electron this center must be capable of extracting electrons from H2O, which results in O2 (click 1). To preserve energy you need a cytochrome system that pumps protons (click 1) and an electron carrier to bring electrons to the cytochromes (click 1). Since the electron’s ultimate destination is NADP+, you need another photosystem to reduce NADP+ to NADPH (click 1). This center must boost the electron to a higher reduction potential. You will need another carrier to bring electrons to this center (click 1). Finally, the protons that are pumped in to the hollow lumen can be used to drive the synthesis of ATP. For that you need an ATP synthase complex (click 1) QH2 + PS II Fd Q Cyt bf PC NADP PSI NADPH + H+ O2 2H2O 4H+ Stroma Lumen CFo ADP + Pi CF1 ATP Before we leave the light reaction, there is just one more important point to consider. The light reaction is supplying the plant with ATP and NADPH. It is important that these two energy sources be balanced. To accomplish this, PSI has a switch that shunts excited electrons back to cytochrome bf. The switch is controlled by the ratio of NADP+/NADPH. To understand the regulation, consider what happens when NADP+ is high (click 1). This means there is plenty of NADP+ to take electrons from ferrodoxin (click 1). But, if NADP+ is low (because NADPH is high), the electron are shunted back to cytochrome bf to make more ATP (click 1). This is how chloroplasts maintain energy balance. NADP+ QH2 PS II Q Fd Cyt bf PC PSI + NADP NADPH NADPH O2 2H2O 4H+ Stroma Lumen CFo ADP + Pi CF1 ATP ATP Test Your Understanding Look at the absorption spectrum of chlorophyll. Can you can see absorption bands representing the 2 photocenters? Yes, but the wavelengths are not exactly at 680 or 700 nm. There are two peaks in and around the near infrared region. Obviously the major absorbing factors in the center at these wavelengths are chlorophyll a and b molecules. The presence of proteins, which are also part of the center will alter the position of the peaks. Why is there only one water-splitting center? Both centers lose electrons. Removing the electron from P680 (PSII) makes the chlorophyll a very powerful oxidant. The power is demonstrated by the ability to pull electrons away from a H2O molecule. P700 (PSI) has the luxury of having an electron from PSII fill the void, and hence water is not needed. Assume one mole of photons struck the P680 center at two wavelengths, 250nm and 700nm. Which will give more energy to the plant, and how much more? 250nm will give more. A mole of photons at 250nm is equivalent to 479 kJ. One mole of photons at 700 nm is equivalent to 171 kJ. At 250 nm , 308 more kJ strike the plant. This computes out to be almost 3 times more energy at the lower wave length of light. See Voet “solutions”, p SP-12