Development
advertisement

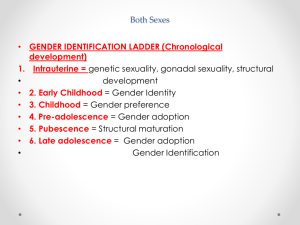
Reproductive endocrinology Gonadal development: Both the testes and the ovaries are derived from the same gonadal primordium. There are two sets of ducts, the Wolfian duct and the Mullarian duct. Development of the primary sexual characteristics depends directly on the endocrine environment during development. An individual can be forced into either a female development or a male development by application of the appropriate hormones, regardless of genetic makeup. In the absence of hormonal stimulation, the gonadal primordium will develop into ovaries and the Mullarian ducts will develop into the uterine ducts, uterus and vagina. Development: The sex organs themselves, along with all their associated ducts and glands are referred to as the Primary sexual characters Secondary sexual characteristics are structures which will enhance reproduction, but are not necessarily required. For example, beard growth in men. Without hormonal stimulation, the Wolffian duct regresses. In males, the gonadal primordium begins to secrete testosterone and Mullarian Inhibiting Substance (MIS). Testosterone stimulates the development of the Wolffian ducts, which subsequently differentiate into the vas deferens, epididymis and seminal vesicles. MIS causes the Mullarian ducts to degenerate Estradiol can prevent MIS from stimulating Mullarian duct regression. Testosterone is converted into dihydrotestosterone (DHT) by the enzyme 5αreductase. DHT influences the development of the external genitalia. The genital tubercle becomes the penis. The genital folds become the shaft of the penis. The genital swellings become the scrotum. Without DHT, the external genitalia are feminized. The genital tubercle becomes the clitorus. The genital folds become the labia minora. The genital swelling becomes the labia majora. MIS is a 140 kDa glycoprotein in the TGF-B superfamily. It activates a Serine-theonine receptor Circulating levels of androgens (and possibly estrogens) also trigger differential development in the brain. Animals exposed to androgens during a specific critical window will develop male reproductive behavior, regardless of the genotype or the physical phenotype. Development of secondary sexual characteristics: This usually coincides with the final maturation of the gonads. In humans, this is referred to as puberty. Mechanism controlling onset is unclear, but appears to involve the loss of inhibition of gonadal development. One potential candidate (at least in males) is melatonin. During childhood, melatonin is produced in the pars intermedia of the pituitary gland. However, after childhood the pars intermedia stops producing melatonin. Melatonin synthesis and secretion are taken over by the pineal gland, but at a much reduced rate. This drastic drop in melatonin secretion (>75%) may trigger the secretion of sex steroids by the adrenal glands and/or the testes. In females, the situation may be different. There is good evidence that the hormone leptin is also involved. Leptin is a hormone released by adipose tissue. Circulating leptin levels may reflect total body fat storage by the body. In females, a certain minimum total-body fat content is required for puberty to progress and for maintenance of the menstrual cycle. Male reproductive system: Spermatogenesis I: The immature germ cell in the male is referred to as the spermatogonium. These cells are located just under the basement membrane of the seminiferous tubules, between adjoining sustentacular (Sertoli) cells. Since sperm production continues throughout adult life and at the peak, 100-200 million sperm can be produced daily, the spermatogonia are constantly renewed. The first step in spermatogenesis is a mitotic division of the spermatogonium. One of the daughter cells remains, to replace the original spermatogonium, while the other cell (now called a primary spermatocyte) undergoes meiosis. Spermatid migration: Spermatogenesis II: The first meiotic division yields two secondary spermatocytes. Usually, these secondary spermatocytes do not fully separate during cell division, leaving a direct cytoplasmic connection between the cells. Following the second meiotic division (again, an incomplete division), the cells are known as spermatids. As the germ cells are undergoing meiosis, they also migrate towards the lumen of the seminiferous tubule. As they approach the lumen, they shed much of their cytoplasm. They are attached to the Sustentacular cells, via specialized junctions, which provide nutrients. When the spermatids reach the lumen, they remain embedded within the sustentacular cells, where they undergo tail development, acrosome formation and nuclear condensation. Finally, the fully-formed spermatozoa are shed into the lumen of the seminiferous tubule, where they are carried to the epididymus. This whole process takes between 60 and 70 days. The end of each ductus deferens (two) enlarges to form ampullae, where sperm are stored until ejaculation. The prostate contains the first part of the urethra (prostatic urethra) which is where the ejaculatory ducts merge with the urethra. The urethra exits the prostate, penetrated the urogenital diaphragm and runs the length of the penis. Male sexual response: Erection The first phase of the male sexual response is erection of the penis, which allows it to penetrate the female vagina. This occurs when the erectile tissue of the penis becomes engorged with blood. When a male is not sexually aroused, the arterioles supplying the erectile tissues are constricted. During sexual excitement, a parasympathetic reflex is triggered that causes these arterioles to dilate (NO2). As a result, the vascular spaces of the penis fill with blood causing the penis to become enlarged and rigid. Expansion of the penis also compresses the veins retarding the outflow of blood and further contributing to the swelling of the penis. This reflex is initiated by a variety of stimuli ranging from thought to touch. Ejaculation A spinal reflex is initiated, producing a sympathetic discharge to the genital organs. As a result, the reproductive ducts and accessory glands contract peristaltically discharging their contents into the urethra. The muscles of the penis undergo a rapid series of contractions propelling semen from the urethra. This is followed by muscular and psychological relaxation and vasoconstriction of the arterioles serving the penis, allowing blood to drain out of the erectile tissue, which subsequently causes the penis to become flaccid again. Role of the Accessory Glands: The seminal vesicles are paired glands that produce about 60% of the semen. Their secretions contain fructose sugar, ascorbic acid and prostaglandins. These are sac shaped glands, approximately 5 centimeters long, which lie along side the ampullae of the ductus deferens. They each empty into a short duct, the ejaculatory duct, which merges with the terminal end of the ductus deferens. These, in turn, fuse with the prostatic urethra which runs from the bladder through the prostate gland. The alkalinity of the fluid serves to neutralize the normally acidic environment in the distal urethra and in the vagina. The fructose is supplied as an energy source for the sperm, and the prostaglandins serve to stimulate smooth muscle contractions in the vagina and cervix. This is thought to facilitate the uptake of sperm into the uterus. The bulbourethral glands are paired glands that secrete a small amount of thick clear mucus. This secretion is released prior to ejaculation and is believed to neutralize traces of acidic urine in the urethra. The prostate gland is a single gland, which secretes about one third of the semen volume. It secretes a milky, slightly acidic fluid containing citrate, acid phosphatase and several proteolytic enzymes. These enzymes are probably involved in breaking down the mucus plug in the cervix. They also appear to contribute to the motility and viability of the sperm Semen Production Remember, Sperm + seminal fluid = semen. Semen provides a transport medium for the sperm. It also provides nutrients for the sperm and chemicals that protect them, activate them and facilitate their movement. The amount of semen released during ejaculation is relatively small, about 2-6 ml but it contains 50-100 million sperm per ml. The proteolytic enzymes are probably involved in breaking down the mucus plug in the cervix. They also appear to contribute to the motility and viability of the sperm. After ejaculation, SgI, SgII and fibronectin aggregate to form a gelatinous mass, which is believed to trap the spermatozoa within the vagina. Liquefaction occurs 5-20 minutes later, through cleavage of the semenogelins by PSA prostatespecific antigen). Brain-testicular axis: Female reproductive system: OOGENESIS I: This process is the equivalent of spermatogenesis in the male. However, the two processes are vastly different. In females, much of the process occurs during fetal development. The primitive germ cells undergo numerous rounds of mitosis, which produces millions of oogonia (2n). Most of these oogonia are resorbed (through a process called atresia). However, a few hundred thousand begin meiosis and enter prophase I. These are now referred to as primary oocytes. OOGENESIS II: There are no oogonia present in the adult female. The primary oocytes are arrested in prophase I and become quiescent until puberty. Cyclical changes in LH and FSH will trigger three or four primary oocytes to finish meiosis each uterine cycle. During the two meiotic divisions, all the cytoplasm will stay with a single daughter cell, which is destined to become the ovum. The other three daughter cells simply develop as small polar bodies that are eventually degraded and resorbed. Fertilization and pregnancy: Implantation: Placental hormones: During early pregnancy, HCG is secreted by the syncitial trophoblasts. Later, the placenta secretes estradiol, progesterone, relaxin and somatomammotropin. Function of placental hormones: HCG is similar to LH (and FSH and TSH) Maintains the corpus luteum in a functional state for 34 months. Shares the common alpha chain. This keeps progesterone levels high and they maintain the functional endometrium. Progesterone keeps the uterine wall intact. Initially the progesterone comes from the corpus luteum in the mother, but eventually the developing placenta secrets its own progesterone. Estrogen initially comes from CL, but then is secreted by placenta. Estrogen softens pelvic ligaments to allow for stretching of the birth canal during delivery. Estrogen increases the sensitivity of the myometrium to mechanical irritation, as well as oxytocin stimulation. Stimulates the expression of oxytocin and prostaglandin receptors. Relaxin is an insulin-like peptide. Relaxin increases flexibility in the pelvic joints. Synergistic with estrogen. Suppressing release of oxytocin. Relaxes smooth muscle of the uterus. Consists of two peptide chains joined with a disulphide bridge. This prevents uterine contractions. Alters collagen in cervix to allow for the stretching during delivery. Somatomammotropin is a protein hormone. Acts like prolactin and triggers the mammary glands to develop. Stimulates development of mammary gland tissue in preparation for lactation. Also stimulates lipolysis in the mother. This mobilizes fatty acids for the embryo. Also triggers elevated plasma glucose in mother, by antagonistic action to insulin. Makes glucose available to embryo. Estrogen is stimulating expression of receptors for oxytocin and prostaglandins. Relaxin is inhibiting uterine contractions. Thus, although the uterus is primed to respond to oxytocin, early labour is inhibited. Late in pregnancy, relaxin levels decrease and the uterus becomes irritable. Induction of labour is still not fully understood, but is believed to be triggered by release of fetal oxytocin. Labour: Towards the end of pregnancy, relaxin secretion falls off, thus, the uterus becomes more sensitive to oxytocin. Initially, the fetus secretes oxytocin into the maternal circulation. The oxytocin stimulates contractions, which push the head down against the cervix. This pressure on the cervix stimulates the release of oxytocin from the maternal pituitary gland. The maternal oxytocin causes more contractions of the uterus, forcing the head of the fetus against the cervix even harder. This is a positive feedback system. Nursing: Two hormones are involved, PRL and oxytocin. PRL stimulates milk production, while oxytocin is required for the expression of milk from the breast. Comparative aspects of sex determination: Mammals have genetically determined sex. Mammals have an X and Y chromosome. Y chromosome confers male characteristics. XX – female: Homogametic sex. XY – male: Heterogametic sex. Homogametic sex is not always female. In birds and urodel amphibians: ZZ is male ZW is female Typically, the homogametic sex is the default sex. The default sex is the one that will develop if NO sex steroid exposure occurs. Chromosomally determined sexual differentiation is referred to as genotypic sex determination (GSD). Not all species with GSD have morphologically distinct sex chromosomes. Temperature-dependent sex determination (TSD): Occurs in a number or reptiles, some teleosts and possibly in some amphibians. Behavioral sex determination (BSD): Most often seen in coral reef fishes. Usually controlled by social situations. Usually seen is populations that have a well-defined social hierarchy. Two major patterns are seen. Protandry: individuals start as males, but change to females. Protogyny: individuals start as female, but change to male. Usually the change is determined by some change is social status. Most common pattern is protogyny. Dominant animal is male, most subordinate animals and female. Reproduction in agnathans: Gonad develops from embryonic cortex, regardless of sex. Circulating levels of sex steroids are very low, due to a lack of binding proteins. Male lampreys: Lobular polycystic testes. Only contain primary spermatocytes. Spermatocytes transform quickly into spawning sperm masses. Interstitial cell masses (between lobules) store cholesterol-positive lipids prior to spawning. Interstitial hydroxysteroid dehydrogenase (3b-HSD) activity increases during spawning. Female Lampreys: Mature follicle rupture shortly before spawning and collect in the coelom. Thecal cell shave elevated 3b-HSD activity and are steroidogenic. Vitellogenesis is stimulated by estrogen. Vitellin and other vitellogenic proteins are synthesized in the liver under the control of estrogen. Endocrine axis in lampreys appears to follow the typical vertebrate plan. Hagfish: Very little is known about deep-sea hagfish. Believed to be able to spawn nor than one time, unlike lampreys. Formation of “pre-ovulatory” corpora lutea and post-ovulatory corpora lutea have been seen. However, the role of these structures is unknown. Don’t see sex change in agnathans; however, some hermaphrodites occur. Usually due to incomplete sex differentiation. Chondrichthyes: See both oviparity and viviparity. All chondrichthyean fish have internal fertilization. Males: Paired testes of the cystic type. Sertoli cells are present but undergo cycling. Cells involved in steroidogenesis and sperm development are resorbed after spawning. Next generation of Sertoli cells is recruited from fibroblasts in the connective tissue surrounding testes. Sertoli cells have relatively high 3b-HSD activity. There are nests of undifferentiated germ cells that proliferate and give rise to spermatogonia each reproductive cycle. Females: Ovary is covered with a germinal epithelium. May contain a large cavity. Cavity is derived from large lymph spaces. As each oocyte matures, a layer of cells around it differentiates into granulosa cells. The cells in the connective tissue covering the oocyte will develop into thecal cells. Again, granulosa cells will develop into pre- and post-ovulatory corpora lutea. Atresia of oocytes is common in selachians Estradiol stimulates vitellogenin synthesis and oviduct growth (where development will occur in viviparous species. In viviparous sharks, the ovaries produx a relaxinlike molecule that is very similar to mammalian relaxin in function. Resembles insulin in form however. Teleost fishes: In the bony fishes we see every possible reproductive strategy. Teleosts arose in the devonian period, approx. 400 million ago. Some groups show GSD, some TSD and many show BSD. Both internal and external fertilization patterns are seen, along with oviparety and viviparety. Also see synchronous and asynchronous breeding patterns. Testosterone 11-keto-testosterone 17,20 b-dihydroxy-4pregnen-3-one Volume of sperm produced Gonadal-somatic index Testosterone Estradiol 17,20 b-dihydroxy-4pregnen-3-one Plasma vitellin gonadotropin Most teleosts have 2 forms of GnRH (1 and 2). Most teleost fish have 2 gonadotrophs. GTH I and GTH II, which don’t structurally resemble LH or FSH. GTH I stimulates synthesis of estrogens. GTH II receptors are only found in the granulosa cells of the follicles. GTH II stimulates production of 17,20 b-dihydroxy4-pregnen-3-one. This enzyme converts some steroids responsible for the final oocyte amturatoin Females: Oviparous and viviparous types. Ovaries are hollow – an adaptation to production of many eggs. Anywhere from 10 – 10,000 at a time. Certainly true in oviparous fish. May not be quite so true for viviparous fish (for example the ceolacanth). Mammalian GnRH and LH will induce ovulation. Mammalian FSH has no effect on ovulation. Males: Testes are of the cystic/lobular type. Cyclical spawners resume spermatogenesis immediately after spawning. Sustentacular cells have been identified in a large number of species. Adapted for production of large volumes of sperm. Have High 3 b HSD activity (produces androgens). Major circulating androgen is 11 keto-testosterone. In many teleosts, the testes lack interstitial cells. There is a different cell type called the lobule boundary cells which secrete some androgen. As leydig cells in mammals. Treating each in turn: 3.) Sex ID is permanent Sequential Hermaphrodites: Sex 1 Sex 2 RS Small Large male female RS Small Large Usually, this produces protogyny (‘female-first’). male female RS Small Large Protogyny: Labroides dimidiata, a cleaner fish on Australian Great Barrier Reef male female RS Small Large Protogyny: Labroides dimidiata, male displays suppress female sex change to male. If he dies (or is removed experimentally), largest female changes. male female RS Small Large Protogyny: Thalassoma bifasciatum, blueheaded wrasse. Lek system with 2 male morphs. Tiny ‘sneaker’ male (permanent). Others = female till big enough to compete as dominant lek males. female RS male Small Large Protandry: Coral reef fish (Amphiprion). Monogamous pairs live under sea anemone tentacles, joined by genderneutral immatures. female RS male Small Large Protandry: Coral reef fish (Amphiprion). If resident female dies (or is experimentally removed), partner switches to female and the largest juvenile becomes male. Reproductive behaviors: Wide range of behaviors exhibited Migration Courtship Nesting Parental care Both sexes can exhibit these behaviors Most behavioral work has been done on males Male behavior can be elicited by injection of androgens. RE Peter, U of Alberta Male behaviors can be abolished by gonadectomy. This also causes regression of secondary sexual charateristics. Courtship behavior: Example: 3 spine stickleback. Normally the males are a non-descript brownishgreen colour dorsally, with a silver belly. During the breeding season, the male develops a red belly. At the same time, the males become aggressively territorial. If the males are castrated less than 1 week prior to nest building, aggressiveness persists for 3-4 weeks. This is followed by nest building. Suggests that territoriality and aggressiveness are not directly controlled by androgens, but initiated by them. If castration is performed more than 1 week before nest building, then all territoriality and aggression is abolished. Diandretic species: As mentioned, have 2 types of males. Primary phase males. Secondary phase males. In many species, the primary phase males are determined genetically, while the secondary phase are sex changed females. The trigger is unknown, but may be behavioral. Prolactin: In fish, prolactin stimulates parental behavior. Usually is seen in controlling male behavior, rather than female. This may be due to difficulty in assessing female behavior. In Discus (Symphysodon nequifasciata), the males secrete mucus which is then eaten by the fry. PRL appears to stimulate nest-building. As mentioned, circulating androgen levels correlate to sperm production, but not necessarily with spawning. Some species will produce sperm in the fall, but spawn in spring. (Several pacific salmon species do this) Not yet known what triggers spawning behaviour. Although photoperiod may be involved, it is not the trigger. Parrot fish spawn every day at sunset (predator avoidance) Seasonal spawners may also respond to photoperiod. Eg. Jenynsia lineata (a poecilid) Transplanted from South America to North America. Normal spawning in southern hemisphere: Jan.-Feb. Transplanted fish spawn in July-Aug. Reproduction in Amphibians: Amphibians have a more complex lifecycle than fish Male Urodels: Testes are of the cystic type. Structurally very similar to teleosts. Germinal epithelia is divided into lobes, then ampullae, then germinal cysts. All the spermatogonia in a cyst mature synchronously. Sertoli cells are recruited from fibroblasts each breeding season. Urodel Testicular Structure As in teleosts, the cells surrounding each lobe consists of lobule boundary cells. These cells undergo seasonal lipid accumulation cycles. Lobule boundary cells and Sertoli cells have 3b-HSD activity, indicating steroidogenesis. Both are probably influenced by gonadotropins. Unlike teleosts, the principle androgen is testosterone (not 11-keto-testosterone). Many are dissociated breeders. Androgen levels are low during breeding, but high during testicular development. Sperm are stored until breeding. In species where secondary sexual characteristics develop, androgens are essential for development. However, androgens alone are not enough. In the newt N. viridescens, nuptial pads develop in response to testosterone and PRL. In addition, several skin glands (which secrete pheromones) are stimulated by both hormones. Unlike teleosts, it is the males that emit pheromones. Complex courtship and mating displays. In both male and female urodels, PRL stimulates migratory behavior known as the “water drive”. Testosterone primes the CNS for mating behavior. After priming, argenine vasotocin and GnRH act synergistically to stimulate clasping behavior. Pheromones from hedonic and cloacal glands stimulate courtship behavior in females. In some species there are “chin” glands which the male rubs over the female. The secretions are believed to contain pheromones which make the female receptive. Abdominal glands release female attractants into the water stream in front of the female In some species there may also be airborne cues released by the females that attract the males. Male anurans: Unlike urodels, the anuran testes is structurally like the testes of the higher vertebrates. They consist of a homogeneous mass of semeniferous tubules. Contain significant numbers of interstitial cells. Interstitial cells have seasonal lipid cycles and have seasonal cycles of 3b-HSD activity. In Bufonid toads, both sexes have ovarian tissue. In the males, the rudimentary ovaian tissue is called the Bidders organ Bidder’s organ is composed of small oocytes. However, these oocytes never reach the vitellogenic stage and never mature. Bidder’s organ undergoes seasonal regression and recrudescence correlated with the testicular cycle. They appear to be steroidogenic as indicated by the elevated 3b-HSD levels. After castration, Bidder’s organ will hypertrophy, but never becomes a functional ovary. Some frog species do undergo sex reversal. Male Apodans: Very little is known about apodan reproduction. Apodans don’t reproduce in water. Internal fertilization and viviparity. Males have an intromittent organ. Males have retained the lower portion of the Mullerian duct, modified into Mullerian glands, which functions somewhat as a prostate, producing seminal fluid. Female Anurans and Urodels: Ovaries are hollow saclike structures. Layer of germinal epithelium composed of granulosa cells with nests of oocytes. There is a very thin layer of thecal cells around each follicle. Granulosa cells contain high levels of 3b-HSD. Corpora lutea form in both oviparous and viviparous species; however, no endocrine role has been identified in oviparous species. Fat bodies: Generally speaking, amphibians invest more in vitellogenesis. Like the teleosts, the liver is the main site of vitellogenic protein synthesis (stimulated by estrogen). However, the fat body is involved in processing the lips that will be incorporated in the yolks. Most amphibians are oviparous and have external fertilization. Two species of European salamanders have true viviparous breeding. Young develop in the posterior portion if the oviduct. Undergo metamorphosis in utero during a 4 year gestation period. Viviparity in urodels is not true viviparity. Usually involves development of a pouch which allows development of the eggs on the body of the adult. Marsupial frogs Swallowing frogs Oviductal incubation. Viviparity is common in Apodans In all 3 groups there is a tendency to reduce the dependency on water. Midwife toad Suriman toad Reproduction in Reptiles: The order is fairly diverse, so generalizations are difficult to make. Also, little work has been done. Different types of sex determination. Genetic sex determination. Temperature-dependent sex determination. Although there are some parthenogenic species, behavioral sex determination is not seen. For all reptiles, fertilization is internal. Males possess an intromittent organ for copulation. Males: Testes structure is similar to mammals. This form is common to all amniotes. Consists of tightly coiled seminiferous tubules. Tubules are composed of germ cells and Sertoli cells. Prominent interstitial cells which are believed to secret androgens. Androgens are responsible for development of secondary sexual characteristics. Females: Have paired ovaries which are hollow. Generally speaking, reptiles lay many fewer eggs than amphibians, but these eggs are heavily yolked. Mainly because the young must emerge fully developed. Follicle is differentiated into an internal thecal layer, the cells of which are granular in appearance, and a fibrous theca externa. Granulosa cells are present but do not appear to be involved in steroid secretion. It is the thecal cells which appear to secrete estrogen. Males: Testes is the same as all other amniotes, a tightly coiled mass of seminiferous tubules. The rest of the ductal system is basically the same as seen in the mammals. In sexually mature squamates, a portion of the kidney tubules undergo a hypertrophic modification. Sperm are stored in these modified tubules. Epithelial cells secrete a fluid which helps maintain the stored sperm until mating occurs. Crocodilians and turtles produce two distinct gonadotropins, while the squamata produce only and FSH-like hormone. The hypothalamus produces GnRH which controls the release of gonadotropins by the pituitary. As in amphibians, there is a distinct fat boby that appears to undergo lipid cycling in synchrony with oogenesis and vitellogenesis. Most reptile show very little in the way of parental behavior, although there are some notable exceptions. Many crocodilians tend the nest and transport the hatching young to the water after emergence. The mother stays with the young and protects them for a significant time period. Many secondary sexual characteristics and reproductive behaviors are stimulated by a combination of androgens and PRL. In Anolis carolinensis have a moderately complex mating pattern. Development of a dewlap, under the chin, is stimulated by androgens. Induction of the red and black colouration is stimulated by PRL The characteristic black eyespots and dewlap spot is intensified by epinephrine. Territoriality is declared by vigorous head bobbing, with extension of the dewlap. In terretoriality displays against other males, circulating EPI levels are elevated. This EPI causes the dewlap spot and the eyespot to darken. In courtship behavior, head bobbing and dewlap displays are also involved. However, EPI is not elevated, therefore the black spots are fainter. Reproduction in Birds: Avian reproduction is on of extremes. All the different aspects of reproduction are greatly modified in consideration of weight reduction. All birds are oviparous. Eggs are heavy and cannot be carried internally for long. Due to complex nature of the avian lifestyle, significant parental investment is the norm. By necessity, birds lay many fewer eggs than reptiles (again, eggs are heavy). Because of this, birds invest in parental care to maximize the survival of so few eggs. Major concessions are made in the reproductive organs in order to conserve weight. Most birds are migratory to some extent, so lugging around fully developed gonads is metabolically foolhardy. Ovarian development: Only one (in most species the right) develops. During the migration of the germ cells, distribution is asymmetric. One primordial ovary has more germ cells than the other. The ovarian cells start to secrete MIF. However, the larger of the two ovaries (the one with the greater number of germ cells) secretes estrogen into the oviduct on that side. This estrogen desensitizes the oviduct (a Mullerian duct derivative) and it will not degenerate. However, the contra lateral oviduct regresses and the smaller ovary is resorbed. Gonadal cycle in males: In all birds, male or female, there is a marked gonadal cycle. In males, this cycle is nothing less than dramatic. Between breeding seasons, the testes almost completely break down. In extreme cases, the regressed testes are 500 times smaller than the fully developed testes. Seasonal testicular development is called recrudescence. There are three phases to the testicular cycle. Photoperiod strongly controls the testicular cycle in most species. Preparatory phase: Begins immediately after the reproductive period. Marked by an almost total collapse of the testes. Males in this phase are insensitive to photoperiodic cues. Referred to as photo-refractory. Testes are little more than sacs of connective tissue with interstitial cells. The end if the preparatory phase is marked by the onset of photosensitivity. The second phase is called the progressive phase. Lengthening photoperiod initiates an increase in activity of the hypothalamic-hypophyseal-testicular axis, resulting in increased gonadotropin secretion. The endocrine basis for photorefractivity is not understood, nor is the induction of photosensitivity. Some evidence suggests that the testosterone surge seen during breeding feeds back on the hypothalamus, causing prolonged suppression of LH. During this phase, there is marked spermatogenesis and increased androgen secretion by interstitial cells. Recrudescing testis Maturing Ovary During this phase, there is increased PRL secretion and there is induction of secondary sexual characteristics and reproductive behavior. For example, territorial displays occur, singing (in songbirds) increases, courtship behavior is induced, etc. There is increased conversion of steroid in the brain (increased aromatase activity can be seen. Brood patches begin to develop. Long periods of cold temperature can block or delay the progressive phase. Aromatase Distribution in Brain Brooding Third phase is the culmination phase. This coincides with ovulation in the females. Usually, the male is ready slightly before the female. Ovulation is probably induced by behavioral cues and/or male-female interactions. Spermiation (release of sperm from testes) can occur once, or several times in rapid succession. Upon completion of the culmination phase, the testes undergo the collapse associated with the preparatory phase onset. Interstitial cells: During the winter, the interstitial cells are small and contain little lipid. Proportionally, they make up a large amount of the testicular volume, simply because the seminiferous tubules are so regressed. During the progressive stage, they become more lipoidal and start to express aromatase activity, reflecting the increase in androgen secretion. At the end of spermeation, there is a rapid depletion in lipid stores. Also at the end of spermeation, there is massive autolysis of the interstitial cells, resulting in an almost complete die off. New interstitial cells are recruited from fibroblasts. Recruitment and hyperplasia are stimulated by LH. Differentiation occurs early on and then the cells lie dormant until the next preparatory phase. Sertoli cells: Do not turn over like the interstitial cells. Have a marked lipid cycle. Become densely lipoidal at the end of breeding and store the lipid until the next round of spermatogenesis. The tremendous growth in the testes results in damage to the tunica albuginea. After each reproductive season, fibroblasts are recruited to build a new tunica albuginea. Female reproductive cycle: Ovarian cycle is not as marked as testicular cycle. There is seasonal regression of the oviducts and assorted glands. Increased estrogen stimulates vitellogenesis. Estrogen also mobilizes calcium. Follicular structure is similar to mammals. However, after ovulation, the ruptured follicle does not reorganize into a corpus luteum. This is probably associated with the total absence of viviparity. The remaining granulosa cells do secrete progesterone, which seems to be associated with regulating movement of the egg through the oviduct and shell gland. FSH stimulates follicular development, as in mammals, while LH stimulates ovulation. Androgens in both sexes: Androgens are important in females as well as males. Generally, if there is a change in plumage associated with reproduction (not always restricted to the males), that change is stimulated by androgens. Androgens inhibit brood patch formation (helps to tightly regulate timing. Aggression and territoriality in males is usually controlled by androgens, but can also be stimulated by FSH (or a combination). Thyroid function in reproduction: Most birds migrate to reproduce. In preparation for migration, there is a spike in thyroid hormone levels. This occurs twice, there is also a spike before the migration to the ‘wintering’ grounds. This first spike is also required in order to support the rapid mobilization of reserves seen in reproduction (birds have very condensed reproductive seasons generally). Control of Gonadotropin Releasing Hormone (GnRH) by the hypothalamus. Release is pulsatile in all mammals looked at (probably true for all vertebrates). Disruption of pulsatile secretion is associated with reproductive disorders in humans. In humans there is only one form of GnRH. Often referred to as LHRH due to sequence homology with one of the two GnRHs found in other vertebrates. In Rhesus monkeys There are about 2000 neurons in hypothalamus that contain LHRH. Early studies have suggested that the pulse generator is physically located in the medialbasal hypothalamus. Critical experiment complete deafferentation of that region does not block pulses. Cells taken from this region and put into cell culture show pulsatile release of GnRH. Pulses are also seen in cultured basal hypothalamus both rat and guinea pig. Electrophysiological evidence also supports the idea that the pulse generator is from this region. Volleys of action potentials (extracellular electrodes) have been recorded in this region and these volleys coincide with the pulses of GnRH. These volleys have only been observed in the basal hypothalamus and nowhere else, suggesting that the pulses do not originate from outside the basal hypothalamus. Pulsatile LHRH release is seen in tissue fragments of median eminence from the rat. This region doesn’t have the cell bodies, suggesting that the pulses are initiated in the axons of the LHRH neurons, not up at the cell bodies. This suggests that other signals must influence LHRH release. One candidate in Neuropeptide Y (NPY). NPY has been shown to stimulate LHRH release from median eminence fragments in culture. NPY is also released in a pulsatile fashion in the stalk median eminence of the rhesus monkey and these pulses appear to be synchronous with LHRH pulses. Note, this is not proof that the NPY is causing LHRH release. There are other possible candidates. γ aminobutyric acid (GABA), dopamine and Nitric Oxide (NO) are also found in the same area and all seem to be released in pulses. Focus on the endogenous pulse generator. LHRH neurons appear to have intrinsic pacemaker activity Some studies have been done on the GT-1 cell line, a mouse line that expresses the mouse GnRH transcript. GT-1 cells release LHRH in pulses with intervals of about 22-30 minutes. These intervals are similar to the intervals seen in the mouse in vivo. This interval is not the same as seen in primates. Primate LHRH neurons in culture also release LHRH in pulses. Interval of around 43-44 minutes. LHRH neurons from 2 sources in rhesus brain had same interval. This suggests that there is an endogenous frequency. Frequency in cultured cells is approximately the same as in adult animals in vivo. In cultured LHRH neurons, electrophysiological studies have demonstrated that they show oscillatory bursting activity (i.e. trains of action potential). However, it is not know for certain if the APs are directly associated with LHRH release. In addition to the electrical activity, cultured LHRH neurons show oscillatory Ca2+ activity. When cultured individually, these neurons all have individual frequencies. However, when cultured together they synchronize their Ca2+ oscillations. The synchronous activity has a frequency similar to the in vivo frequency. A similar system of activity has been shown to correlate to insulin release in β cells. However, a link still needs to be established in LHRH neurons. Mechanism of communication between LHRH neurons: Possibilities: Some sort of synaptic transmission? Electrical coupling (i.e. gap junctions)? Some diffusible messenger (i.e. paracrine signaling)? GT-1 cells have been demonstrated to form both synapses and gap junctions in culture. However, it has been shown that LHRH neurons grown on separate coverslips (no physical contact) also synchronize. Thus, we can rule out synaptic transmission and gap junctions as potential methods of cell communication. This leaves paracrine signaling. NO synthase mRNA has been found in GT-1 cells. NO would be an ideal candidate. Small molecule. Diffuses rapidly. Highly soluble. Penetrates membranes easily. In LHRH neurons: Show characteristics of neuroendocrine cells. Depolarization causes release of LHRH (induced depolarization). Depolarization also causes Ca2+ oscillations. In GT-1 cells: Depolarization by high extracellular K+ causes LHRH release. Treatment with Veratridine (VG Na+ channel opener) causes LHRH release. Both these treatments also cause LHRH release from cultured fetal rhesus monkey LHRH neurons. Na+ channel blockers (i.e. tetrodotoxin) will block LHRH release. LHRH release is dependent on Ca2+ influx. Suggests the possibility that Ca2+ influx may be involved in the pulse generation (rather than being a result of pulse generation). L-type Ca2+ channels have been found in GT-1 cells as well as monkey LHRH cells. This is a voltage-gated Ca2+ channel. Characterized by nifedipine blockade, but not blockade by ώ conotoxin. Also, they are activated by Bay-K8644. Bay-K8644 does stimulate LHRH release in both cell systems. L-type channels have been shown to be involved in Ca2+activated Ca2+ influx. Ca2+-activated Ca2+ influx has been identified in GT-1 cells. Treatment with thapsigargan or cyanide-p-trifluoromethoxyphenyl-hydrazone (FCCP; induces Ca2+ release from mitochondria) increased [Ca2+]i and induced oscillations. Similar treatments (i.e. thapsigargan or ryanodine) induced calcium oscillations in fetal monkey LHRH neurons. Other potential influences: Neuropeptide Y (NPY) 36 aa peptide These studies were done in vivo on monkeys, using the push-pull cannula technique. Perfusate In Under pressure Effluent out Skull Push-pull cannula Doublebarreled cannula Perfusate percolates Through interstitial space Infusion (8 hour) of antisense oligonucleotide against NPY blocks both the NPY pulses and LHRH pulses. Infusion of anti-NPY antisera will also block both LHRH and NPY pulses. Thus, good evidence that NPY is playing a role in at least regulating the LHRH pulses, but is it the only factor? Norepinephrine (NE) may also be involved. There are NE containing neurons in the same region of the stalk median eminence. Adrenergic input has been shown to modulate LH and LHRH release. LH pulses (from pituitary) and intermittent bursts of multiple unit activity (in ME) occur in close association with LHRH pulses and can be suppressed with α-adrenergic blockers phentolamine and prazosin (α1-specific). Push-pull cannula results show that release of NE from stalk-ME is pulsatile and the pulses are synchronous with LHRH pulses. Direct infusion of NE, or α-adrenergic agonists, into stalk-ME stimulates release of LHRH. The α1-blocker prazosin reduces pulse amplitude, but not pulse frequency. β- and α2- antagonists have no effect. Infusion of NE into stalk-ME also stimulates the release of prostaglandin E2 (PGE2). PGE2 infusion will also stimulate LHRH release. Thus, NE may work directly, or through PGE2 , or both. Organ culture on ME fragments has shown that NE effects are mediated through PGE2 . Interactions between NPY and NE/ PGE2 NPY infused into stalk-ME of prazosin-treated monkeys stimulated LHRH release. This suggests that NPY is working independently of NE/ PGE2. Also, α1- adrenergic stimulation with methoxyamine in monkeys treated with antisense oligonucleotide for NPY still had LHRH release suggesting that NE effects are not working through NPY neurons. Thus, it appears that NPY and NE are working independently to modulate LHRH release. γ-aminobutyric acid (GABA) This is the major inhibitory neurotransmitter in the CNS and it is found in the hypothalamus. Early studies, involving infusion of GABA into the CSF in the third ventricle, stimulated LHRH release This was also observed in explants of hypothalamic tissue kept in culture. However, other studies were contradictory. A more recent observation is that GABA may change in activity with changing age or developmental status. In rats, GABA appears to stimulate LHRH release in juveniles, but becomes inhibitory at puberty. More recently, studies of GT-1 cells and LHRH neurons (from mouse olfactory placode) show that GABA actually stimulates LHRH release, as well as increasing intracellular Ca2+ oscillations and membrane potentials (depolarization). In monkeys (using the push-pull cannula)… GABA tonically inhibits LHRH release before puberty. GABA levels in ME are much higher in prepubertal animals and levels drop by mid-puberty Another neurotransmitter found in the ME region is glutamate. It is an agonist of the excitatory amino acid system, working on NMDA receptors. It has also been shown to stimulate LHRH release. Stimulation of N-methyl-D-aspartate (NMDA) receptors can induce precocious puberty in rats. There are potential interactions between GABA and glutamate. GABA is synthesized from glutamate. This raises the possibility that GABA and glutamate levels may be related. Infusion of antisense oligonucleotides for enzymes involved in the synthesis of GABA from glutamate cause an increase in LHRH in pre-pubertal monkeys. i.e. when GABA synthesis was inhibited, LHRH levels rose. Was this due to a local increase of glutamate, once GABA synthesis was stopped? Or, did reduction of GABA simply unmask an ongoing glutamate stimulation? Several studies suggest the former. In pre-pubertal monkeys, the glutamate levels in the stalk ME are very low. When GABA synthesis is blocked, these levels rise. However, there are studies that suggest the former may also be occurring. The probability is that both occur to some extent (and there may be variability between species). As mentioned before, NO may be playing a role and is an ideal candidate. Pulsatile LHRH release occurs even when the cell bodies are not present. This suggests some form of pre-synaptic stimulation at the neuroterminals of the LHRH neurons. Histological studies have failed to show the presence of pre-synaptic synapses in the right area of the hypothalamus. Similar results are seen in cultures cells. Cells that are physically separated, yet cultured in the same dish, show synchrony of LHRH pulses and Ca2+ oscillations. This is strong evidence that there is a chemical mediator (i.e.some kind of paracrine signaling), which coordinates the LHRH release. In push-pull cannula experiments, infusion of the NO precursor L-argenine stimulated both NPY and LHRH. Infusion of D-argenine had no effect (the dextrorotary form cannot be converted into NO). The enzymes involved in NO synthesis are present in an adjacent area of the hypothalamus. This means that NO is available in the area in question. Finally, glial cells may also be playing a role. In other systems, glial cells have been shown to modify, or affect the release of neurotransmitters and neurohormones. In this system, circumstantial evidence suggests this possibility. The endogenous pacemaker seems to be located in, or near the neuroterminals of the LHRH cells (and not near the cells bodies). No pre-synaptic synaptic connections have been identified in the area. Glial cells ARE present around the neuroterminals. It is known that glial cells play an important role in regulating release of the hormones of the pars nervosa, an analogous system to that of the stalk ME. The proposed mechanism for this interaction is that glial cells may release the kallekrein bradykinin. Bradykinin, in turn, would stimulate glutamate release from astrocytes located around the neuroterminals. This would have an effect on LHRH release from the neuroterminals themselves. NPY NE LHRH GABA GABA Glu Glu NO Master Pacemaker? GABA Glia Glia Portal blood Have a good summer!