What Is A Prodrug?
advertisement

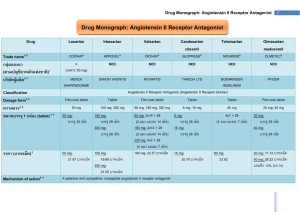
PRO DRUGS: OLMESARTAN MEDOXOMIL Kelsey Davis, Courtney Getchell, Robert Kolar Introduction We will be covering the topic of prodrugs, specifically a hypertension medication Olmesartan. 1) An introduction to prodrugs and what they are used for. 2) How Olmesartan works and is distributed. 3) Benefits and Risks. What Is A Prodrug? Simply, a prodrug is the precursor of the active form of a drug. In more complex terms, a prodrug is an inactive form of a drug, but once administered, it undergoes a conversion by metabolic processes to become the active, pharmacological agent. Why Design & Prescribe Prodrugs? Prodrugs are designed to improve UNDESIRABLE and CHALLENGING properties of drugs such as: Prodrugs ENHANCE the ADAME properties of the drugs. Especially: Formulation Delivery Toxicity Absorption Distribution Metabolism Excretion And, Prodrugs are designed with greater selectivity for a particular target. This improves the effect of the drug and also contributes to decreasing the risk of unwanted tissue and organ toxicity. Prevalence of Prodrugs in Medicine Surprisingly, prodrugs are found in numerous types of medications. The beginning of the 21st century marked breakthroughs in the development of prodrugs. Since 2008, at least 33% of small-molecular-weight drugs have been prepared as prodrugs. Currently, 10% of all marked medicines are classified as prodrugs. Structure of a Prodrug Generally, a prodrug is comprised of three key components: The promoiety, the parent molecule, and the spacer. The promoiety is a molecule used to mask the active group of the drug by bonding to the parent molecule. The parent molecule is the component that is released from the promoiety once it is inside the body and contains the active form of the drug. The spacer is a synthetic handle used to attach the drug and the promoiety, especially in the case of steric hindrance. In some cases, a double prodrug may be prepared. This consists of a second promoiety attached to the first promoiety linked to the parent drug. These promoieties are usually different and require different methods of hydrolysis. Prodrug Structures Single Prodrug Spacer Double Prodrug Bioactivation of a Prodrug Once administered the body, prodrugs can be converted to the active form by chemical reactions of metabolic processes or more commonly by enzymemediated processes. The enzymes involved in bioactivation largely include: Esterases hydrolyze esters into two components: an acid and an alcohol. Cytochrome P450 Enzymes perform an oxidation reaction of an R-group, converting it to an alcohol In terms of prodrug activation, these Cytochrome P450 enzymes oxidize the prodrug, resulting in the production of a metabolite, or the active parent drug. General Mechanism of a Prodrug 1. 2. 3. 4. 5. The prodrug, containing its parent molecule and promoiety, is administered to the body. It remains in that form while in the extracellular fluids and while crossing barriers to reach its target. Once at the site of action, conversion of the prodrug will take place either by chemical or enzymatic reactions. The prodrug is disassembled into its parent molecule (active drug) and the promoiety. The parent molecule releases the active drug particles and the promoiety leaves the cell or tissue and is excreted. Bioactivation: Olmesartan Medoxomil Due to the nature of prodrugs, they must be bioactivated to be useful. Olmesartan Medoxomil is converted to its active form in the intestines when it is absorbed. During absorption, ester hydrolysis activates olmesartan medoxomil to the active form olmesartan Ester Hydrolysis • Utilizing enzyme activity, olmesartan medoxomil is converted to olmesartan by addition of water and release of carbon dioxide and diacetyl. • There is an addition of an OH group where the diacetyl is taken off . This is seen with most CYP activity and is useful for helping the body excrete the toxin. Absorption Using suspension of the tablet in a formula solution or tablets produce the same effect. Taking olmasartan medoxomil with food does not change the effective dose of olmasartan. In the Blood Stream After being hydrolized by ester hydrolysis, Olmesartan is not metabolized any further. Blood concentration levels stay at a steady 26% Within 1-2 hours, the concentration of olmasartan in the blood is at its peak. Distribution Olmasartan binds effectively to blood plasma protein, but does not penetrate red blood cells. This allows for effective transport throughout the body Travelling through the body, it stays in the blood stream and targets smooth muscle angiotensin receptors. Olmasartan poorly penetrates the blood brain barrier, if at all. Regulation of Blood Pressure Baroreceptors sense increased arterial pressure (case of high blood pressure) 2. Increased action potentials sent to the vasomotor system in the medulla via the glossopharyngeal nerve 3. Signal inhibits the vasoconstriction center (sympathetic) and excites vasodilation (vagal parasympathetic) centers sends message using ACh as neurotransmitter along the vagus nerve 4. Results in reduced heart rate, cardiac output, contractile strength, and peripheral resistance, which contribute to lowering blood pressure * Opposite stimulation and inhibition occurs when blood pressure is lowered, sympathetic system uses norepinephrine as a neurotransmitter instead of ACh 1. The Role of Angiotensin Angiotensinogen is released in liver, converted to angiotensin I by Renin from the kidneys, and then converted to angiotensin II by angiotensin converting enzyme (ACE) originating from the lungs and kidneys. Angiotensin II increases heart rate via SNS stimulation and also increases water retention via ADH and aldosterone. This leads to increased blood pressure. Renin is released to enable the Renin-Angiotensin System (RAS) during low arterial pressure conditions2 Hypertension Sustained high blood pressure (>140/90) Increased chance of heart disease, coronary artery disease, renal failure, and stroke Caused by increased peripheral resistance Disruption of sympathetic nervous system and/or the reninangiotensin-aldosterone system Increased risk through obesity, smoking, high sodium diet, sedentary lifestyle, stress, and genetic propensity Hypertension and Angiotensin Parasympathetic effects of baroreceptors are diminished during hypertension because they specialize in short-term regulation Hypertension affects kidney function by restricting renal blood flow and preventing regular resorption Excess salt retention increased RAS activation Renin is released--> Angiotensin I forms and is then converted to Angiotensin II Binds to receptor, increasing heart rate and aldosterone release Excess fluid retention from ADH and aldosterone Fluid balance returns to normal, but arterial pressure rises via increased sympathetic stimulation and fluid retention Olmesartan Mechanism of Action Angiotensin II is formed by ACE and then binds to AT1 receptors in vascular smooth muscle Olmesartan blocks this receptor (and AT2 to a lesser extent) Prevent binding of angiotensin II, and therefore its vasoconstriction action is inhibited lower blood pressure Aldosterone release is not stimulated by angiotensin II decreased water retention reduced blood volume Other effects of Olmesartan Negative feedback of angiotensin II on renin production is inhibited Renin and angiotensin II level remain high This has no major effect, as the receptors are blocked Contrary to ACE inhibitors, angiotensin receptor blockers do not inhibit the conversion of bradykinin to inactive kinins Bradykinin induces vasodilation and respiratory constriction (inflammatory peptide) Pharmokinetics Follows linear pharmokinetics for single doses <320mg and multiple doses of 80 mg The dosage declines at a constant rate Half life independent from concentration Clearance independent of dose Olmesartan has a plasma clearance of 1.3 L per hour and a renal clearance of 0.6 L per hour11 Clearance= Volume of distribution * K (elimination constant) Rate of removal from plasma and kidneys Excretion After converted to its active form, no further metabolism occurs to Olmesartan Two phase elimination with a half-life of 13 hours in the second phase 35% to 50% is resorbed from urine, and the rest is excreted via the biliary system (feces) Benefits Activation by our own metabolism allows for an inactive drug with little risks to be administered. Damaging effects can be seen from active drugs being ingested, such as ibuprofen and its tendency to cause ulcers when used heavily Use of our bodies metabolism allows the detoxifying effects to be lessened, allowing for higher levels of bioavailable drug. Olmesartan can be administered at an 80mg dose which is double the dosage point where there is no increased effect and not be dangerous. It also can be administered effectively at low doses, approximately 2.5mg Benefits Continued Excretion of Olmesartan is slow enough to allow a 40mg dose to be 90% effective at 24 hours after ingestion. Once daily administration is sufficient to provide 24 hours of medication effect It only takes 1-2 hours for peak availability Risks Renal impaired patients cannot excrete Olmesartan readily and can see tripled blood serum levels after multiple doses. Use in pregnancy and infants younger than a year old show signs of abnormal kidney growth and high morbidity due to RAAS pathway being blocked. This is associated with all renin-angiotensin system drugs Side Effects Common side effects of Benicar include dizziness, bronchitis, back pain, headache, flu-like symptoms, hematuria, and sinusitis. Sources 1. "Benicar (Olmesartan Medoxomil) Drug Information: Clinical Pharmacology - Prescribing Information at RxList." RxList. 27 June 2014. Web. 24 Apr. 2015. . 2. Huttunen, K.M., Raunio, H., and Rautio, J. Prodrugs---from Serendipity to Rational Design. Pharmacological Reviews. 2011; 63: 750-771. 3. 4. 5. 6. 7. 8. 9. 10. Klabunde R. Arterial Baroreceptors. Cardiovascular Physiology Concepts. April 24, 2014. http://www.cvphysiology.com/Blood%20Pressure/BP012.htm. Accessed April 18, 2015. Paul M, Mehr A, Kretz R. Physiology of Local Renin-Angiotensin Systems. Physiological Reviews. July 2006;86(3):747-803. Whelton PK, He J, Appel LJ, et al. Primary Prevention of Hypertension: Clinical and Public Health Advisory From the National High Blood Pressure Education Program. JAMA. 2002;288(15):1882-1888. doi:10.1001/jama.288.15.1882. Navar LG. Counterpoint: Activation of the Intrarenal Renin-Angiotensin System is the Dominant Contributor to Systemic Hypertension. Journal of Applied Physiology. 2010;109(6):1998-2000. doi:10.1152/japplphysiol.00182.2010a. Head G. Baroreflexes and cardiovascular regulation in hypertension. Journal of Cardiovascular Pharmacology. 1995;26(2):S7-16. Kobori H, Nangaku M, Navar L, Nishiyama A. The Intrarenal Renin-Angiotensin System: From Physiology to the Pathobiology of Hypertension and Kidney Disease. Pharmacological Reviews. September 2007;59(3):251-287. Navar LG. Counterpoint: Activation of the Intrarenal Renin-Angiotensin System is the Dominant Contributor to Systemic Hypertension. Journal of Applied Physiology. 2010;109(6):1998-2000. doi:10.1152/japplphysiol.00182.2010a. Klabunde R. Arterial Baroreceptors. Cardiovascular Physiology Concepts. April 24, 2014. http://www.cvphysiology.com/Blood%20Pressure/BP012.htm. Accessed April 18, 2015. 11. Paul M, Mehr A, Kretz R. Physiology of Local Renin-Angiotensin Systems. Physiological Reviews. July 2006;86(3):747-803. 12. Prodrug. (n.d.) Dorland's Medical Dictionary for Health Consumers. 2007. Web. 20 Apr. 2015. 13. "Promoiety." Promoiety. ProZ.com. 15 Apr. 2011. Web. 17 Apr. 2015 14. 15. 16. 17. Ratain MJ, Plunkett WK Jr. Principles of Pharmacokinetics. In: Kufe DW, Pollock RE, Weichselbaum RR, et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. Head G. Baroreflexes and cardiovascular regulation in hypertension. Journal of Cardiovascular Pharmacology. 1995;26(2):S7-16. Kobori H, Nangaku M, Navar L, Nishiyama A. The Intrarenal Renin-Angiotensin System: From Physiology to the Pathobiology of Hypertension and Kidney Disease. Pharmacological Reviews. September 2007;59(3):251-287. Yang, Y., Aloysius, H., Inoyama, D., Chen, Y., and Hu, L. Enzyme-mediated hydrolytic activation of prodrugs. Acta Pharmaceutica Sinica B. 2011; 1(3): 143-159.