Drug Monograph ARB
advertisement
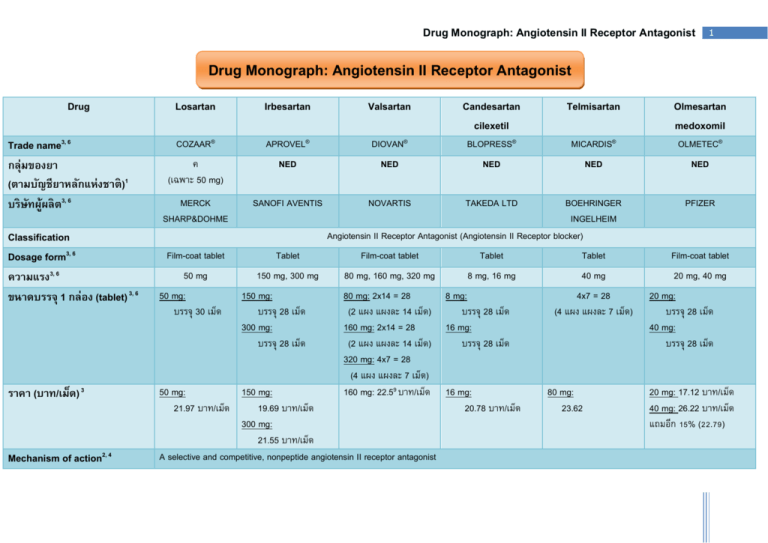
Drug Monograph: Angiotensin II Receptor Antagonist 1 Drug Monograph: Angiotensin II Receptor Antagonist Drug Trade name3, 6 กลุ่มของยา (ตามบัญชียาหลักแห่งชาติ )1 บริ ษทั ผู้ผลิ ต3, 6 Classification Dosage form3, 6 ความแรง3, 6 ขนาดบรรจุ 1 กล่อง (tablet) 3, 6 ราคา (บาท/เม็ด) 3 Mechanism of action2, 4 Losartan Irbesartan Valsartan Candesartan cilexetil Telmisartan Olmesartan medoxomil COZAAR® APROVEL® DIOVAN® BLOPRESS® MICARDIS® OLMETEC® ค (เฉพาะ 50 mg) NED NED NED NED NED MERCK SHARP&DOHME SANOFI AVENTIS NOVARTIS TAKEDA LTD Film-coat tablet Tablet Film-coat tablet Tablet Tablet Film-coat tablet 50 mg 150 mg, 300 mg 80 mg, 160 mg, 320 mg 8 mg, 16 mg 40 mg 20 mg, 40 mg 50 mg: บรรจุ 30 เม็ด 150 mg: บรรจุ 28 เม็ด 300 mg: บรรจุ 28 เม็ด BOEHRINGER INGELHEIM Angiotensin II Receptor Antagonist (Angiotensin II Receptor blocker) 80 mg: 2x14 = 28 (2 แผง แผงละ 14 เม็ด) 160 mg: 2x14 = 28 (2 แผง แผงละ 14 เม็ด) 320 mg: 4x7 = 28 (4 แผง แผงละ 7 เม็ด) 160 mg: 22.59 บาท/เม็ด 8 mg: บรรจุ 28 เม็ด 16 mg: บรรจุ 28 เม็ด 50 mg: 150 mg: 16 mg: 21.97 บาท/เม็ด 19.69 บาท/เม็ด 20.78 บาท/เม็ด 300 mg: 21.55 บาท/เม็ด A selective and competitive, nonpeptide angiotensin II receptor antagonist 4x7 = 28 (4 แผง แผงละ 7 เม็ด) 80 mg: 23.62 PFIZER 20 mg: บรรจุ 28 เม็ด 40 mg: บรรจุ 28 เม็ด 20 mg: 17.12 บาท/เม็ด 40 mg: 26.22 บาท/เม็ด แถมอีก 15% (22.79) Drug Monograph: Angiotensin II Receptor Antagonist Drug Indication USFDA Approval 4 Non-USFDA Approval 4 Losartan Irbesartan Hypertension Hypertension Diabetic Diabetic nephropathy nephropathy Cerebrovascular accident, In hypertensive patients with left ventricular hypertrophy; Prophylaxis Cardiovascular Atrial fibrillation event risk, (Class IIb, Category Reduction (Class B) IIa, Category B) Congestive heart Congestive heart failure (Class IIb, failure (Class IIa, Category B) Category B) Left ventricular Left ventricular hypertrophy (Class hypertrophy (Class IIb, Category B) IIb, Category B) Valsartan Candesartan cilexetil Telmisartan Hypertension Hypertension Congestive heart failure Myocardial infarction Hypertension Heart failure Diabetic nephropathy (Class IIa, Category B) Left ventricular hypertrophy (Class IIb, Category B) Cerebrovascular Congestive heart accident; Prophylaxis failure (Class III, (Class IIb, Category Category B) B) Left ventricular Diabetic nephropathy hypertrophy (Class (Class IIa, Category IIb, Category B) B) Left ventricular hypertrophy (Class IIb, Category B) 2 Olmesartan Medoxomil Hypertension Drug Monograph: Angiotensin II Receptor Antagonist Drug Losartan 3 Irbesartan Valsartan Candesartan cilexetil Telmisartan Olmesartan Medoxomil 2 hours 2 hours 2 - 4 hours within 3 hours 1 week 6 hours 3 – 6 hours 4 - 6 hours 6 - 8 hours - 2 - 4 weeks 3 - 6 weeks 2 - 4 weeks clinical response within 2 weeks - - 6-8 hr Multiple Dose: 24 hr 25% Single Dose: at least 24 hr Single Dose: at least 24 hr Multiple Dose: up to 7 days 25%-35% clinical response in 1 - 2 week Control of blood pressure is sustained more than one year Single Dose: 24 hr Multiple Dose: 24 hr 60%-80% 15% 42% 24 hr Multiple Dose: 24 hr 26% No No 40%-48% in AUC No No No 98.7% 90% 94%-95% > 99% > 99.5% 99% 34 L 53-93 L 17 L 0.13 L/kg 500 L 15-40 L Pharmacokinetic 4 Onset (hours): Antihypertensive, oral Initial Response: Antihypertensive, oral Peak Response: Antihypertensive, oral Maximal effect Duration (hours): Hypertension Absorption (Bioavailability) Food interactions Distribution Protein Binding Volume of distribution Multiple Dose: 24 hr Single Dose: Multiple Dose: 24 hr Single Dose: Drug Monograph: Angiotensin II Receptor Antagonist Drug Metabolism Prodrug Active metabolite Elimination: fecal Elimination: urinary Elimination half-life (hours) Contraindication 4 Precaution 4 4 Losartan Irbesartan Valsartan Candesartan cilexetil Telmisartan Olmesartan Medoxomil Liver, 14% (CYP450-2C9 and 3A4) - Liver, 50%-70% (CYP450 2C9) Hepatic metabolism Intestinal wall cell (CYP450-2C9) liver, less than 3% conjugation De-esterification - - Candesartan cilexetil - Olmesartan EXP 3174 - - Candesartan - Olmesartan 60% 80% 83% 67% 97% 50-65% 35% 20% 13% 33% < 1% 35-50% 1.5-2 11-15 6-9 3.5-4 24 13 angioedema aortic, mitral valve, or renal artery stenosis concomitant use with an ACE inhibitor and a betablocker is not recommended in CHF patients geriatric patients 75 years or older with heart failure heart failure hepatic or renal impairment hyperkalemia congestive heart failure, severe dialysis hepatic impairment or biliary obstructive disorder renal artery stenosis, unilateral or bilateral renal impairment angioedema excessive hypotension - volume-depletion hyperkalemia renal artery stenosis severe CHF แพ้ยา หญิงมีครรภ์ angioedema excessive hypotension volume-depletion hepatic or renal impairment hyperkalemia renal artery stenosis angioedema excessive hypotension hepatic or renal impairment hyperkalemia hypertrophic cardiomyopathy Drug Monograph: Angiotensin II Receptor Antagonist Drug Losartan Irbesartan severe CHF Adverse Reactions4, 5 Cardiovascular Chest pain 12% hypotension 7% Central nervous system Fatigue 14% Dizziness 4% Endocrine Hyperkalemia 1-7% Hypoglycemia 14 % Valsartan Candesartan cilexetil Telmisartan excessive hypotension volume-depletion hepatic or renal impairment hyperkalemia severe CHF surgery/anesthesia Volume- and/or salt-depleted patients volume depletion Orthostatic hypotension 5% hypotension 6-7% Postural hypotension 2% hypotension 19% Fatigue 4% Dizziness 10% Fatigue 2-3% Dizziness 2-17% Dizziness headache Chest pain 1% hypotension 1% Peripheral edema 1% headache 1% Dizziness 1% Fatigue 1% Hyperkalemia 19% Hyperkalemia <1-2% Hyperkalemia <1-6% 5 Olmesartan Medoxomil Dizziness 3% Hyperglycemia hypertriglyceridemia Drug Monograph: Angiotensin II Receptor Antagonist Drug Gastrointestinal Hematologic Neuromuscular and skeletal Respiratory Pregnancy category 4 Breast feeding 4 Losartan Diarrhea 2-15% Gastritis 5% Anemia 14% Irbesartan Diarrhea 3% Dyspepsia 2% Weakness 14% Back pain 2-12% Cough: similar to placebo Cough: similar to placebo Valsartan Candesartan cilexetil Telmisartan 6 Olmesartan Medoxomil Diarrhea 5% Abdominal pain 2% Neutropenia 2% Dyspepsia Gastritis Diarrhea 3% Dyspepsia 1% Diarrhea Back pain 3% Arthralgia 3% Back pain Weakness Back pain 3% Myalgia 1% Back pain CPK increased Upper respiratory infection 7% Rhinitis sinusitis Cough 3% Dyspnea rhiitis Category C (1st Trimester) Category D (2nd Trimester) Category D (3rd Trimester) Infant risk cannot be ruled out Drug Monograph: Angiotensin II Receptor Antagonist Reference 1. คณะกรรมการแห่งชาติดา้ นยา. บัญชียาหลักแห่งชาติ พ.ศ. 2551 (แก้ไขเพิม่ เติมครัง้ ที3่ ). 2008;[79 screens]. Available at: URL:http://wwwapp1.fda.moph.go.th/ed2547/ed-list.asp Accessed May 16, 2009. 2. สมชาย เอีย่ มอ่อง, สุรพันธ์ สิทธิสขุ และสมเกียรติ. Angiotensin II Receptor Blocker. พิมพ์ครัง้ ที่ 1. Text and Journal Publication Co. Ltd. Bangkok 2546. 3. ศูนย์ขอ้ มูลข่าวสารด้านเวชภัณฑ์ กระทรวงสาธารณสุข ราคาอ้างอิ งของยาเดือนตุลาคม-ธันวาคม 2551. 2008 [160 screens]. Available at: http://www.dmsc.moph.go.th Accessed May 16, 2009. 4. Klasco RK (ed): DRUGDEX® System. Thomson Micromedex, Greenwood Village, Colorado (Edition expires March 2009). 5. Lacy,C.F., Armstrong,L.L., Goldman,M.P. & Lance,L.L. Drug Information Handbook International with Canadian and International Drug monographs. 14th ed. Ohio: Lexi-Comp, Inc.; 2006. 6. Romano MB. ed. MIMS Thailand 1st Issue 2008. 104th ed. Bangkok: TIMS (Thailand) Ltd; 2008:55-56. 7