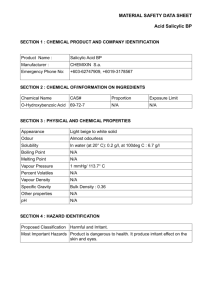
Figure 1: The solubility of salicylic acid in the following
advertisement

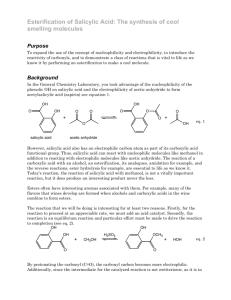
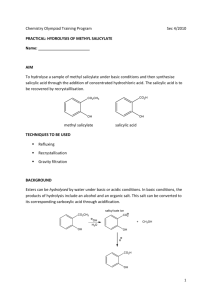
Salicylic Acid Synthesis of Salicylic Acid and it’s Derivatives, their applications, And the Importance of the Ortho-directing group – from an Organic Perspective Presented in fulfillment of requirements in CHEM 411, Dr. Xiaoping Sun, 2014 1 Salicylic Acid 2 Salicylic acid has a variety of functions in plant life, cosmetics, and agricultural products. Derivatives of salicylic acid add another copious range of uses. Salicylic acid is extensively used in the synthesis and production of aspirin - whose medical uses include easing aches, pains, reducing fevers, and serves as an antiinflammatory agent – as well used in a variety of commercial applications. The formula for salicylic acid is C6H4(OH)COOH, where the carboxyl group is ortho to the –OH group. There are multiple means of commercial synthesis of salicylic acid; one of them is crystallization and is achieved in the final step of synthesis. Crystallization is an important step in many aspects of the synthesis because it determines the yield and quality of the target product. Finding the solubility of salicylic acid in different solvents is necessary to determine optimal solvents to use in the production process (Shalmashi & Eliassi, 2008). The solubility of salicylic acid in water, ethyl acetate, carbon tetrachloride, ethanol, and xylene was measured through gravimetrical analysis and reported from temperatures 298 to 348 K. Salicylic acid’s solubility decreases in the order of ethanol, ethyl acetate, carbon tetrachloride, xylene, and water as seen in Figure 1 below (Shalmashi & Eliassi, 2008). Salicylic Acid Figure 1: The solubility of salicylic acid in the following solvents is listed in increasing order: water, carbon tetrachloride, xylene, ethyl acetate, and ethanol. (Shalmashi & Eliassi, 2008) However, another methodology of synthesis, and arguably the most profitable method, is through the Kolbe-Schmitt reaction, named after Hermann Kolbe and Rudolf Schmitt. This reaction is a carboxylation chemical reaction, which incorporates the heating of the reactant with carbon dioxide under pressure (100 atm, 125 °C), followed by presenting the product with sulfuric acid (Taber, 2011). The overall mechanism of synthesis for medical and cosmetic uses is shown below in the Kolbe-Schmitt reaction: 3 Salicylic Acid 4 Figure 2: The outlined mechanism for commercial salicylic acid synthesis via the Kolbe-Schmitt reaction. The reaction starts with phenol and sodium hydroxide under 100 atm of pressurized CO2. (Taber, 2011). When salicylic acid is used directly on the human body, it causes gastric pain because it attacks the mucous membranes of the mouth and esophagus (Williamson, 1994). For the medical market, synthesis of salicylic acid is only a meager accomplishment. The medical uses of salicylic acid are achieved through its derivative, acetylsalicylic acid, commonly known as aspirin – an anti-inflammatory agent, an antipyretic (fever reducer), and an analgesic (painkiller) (Williamson, 1994). Salicylic Acid 5 Acetylsalicylic acid’s capacity to act as an anti-inflammatory agent is due to the fact that serves in the inhibition of the synthesis of prostaglandins, C-20 molecules that increase inflammation. By moving the acetyl group on a terminal amine group on the enzyme, aspirin alters the oxygenase activity of the prostaglandin synthetase (Williamson, 1994). The synthesis of acetylsalicylic acid is achieved through reacting with acetic anhydride in the presence of H3PO4 as shown below: Figure 3: Overall reaction involving of synthesis of aspirin. (Williamson, 1994) Additionally, salicylic acid has paramount uses in plant life. It serves as a crucial regulator of directed plant resistance towards pathogens (Metraux, 2010). The salicylic acid functions range throughout the plant kingdom for physiological processes like thermogenesis or defense against destructive or harmful pathogenic microorganisms. Salicylic Acid 6 Recognition of the pathogen by elicitors released at the site of infection is rapidly followed by changes in ion fluxes and production of reactive oxygen species that starts a signaling cascade leading to the activation of transcription factors involved in the activation of defense genes. Such genes function in the synthesis of regulators such as salicylic acid or ethylene, cell wall strengthening, production of antibiotic metabolites or in the development of a hypersensitive reaction. (Metraux, 2010, p. 1) This signaling cascade leads to local acquired resistance (LAR) in the immediate area surrounding the infected cells. Furthermore, defense reactions are also utilized at locations other than the initial infection site, resulting in a systemic acquired resistance (SAR). Transgenic or mutated plants damaged without the accumulation of salicylic acid cannot produce sufficient defense responses to pathogens once an infection is present – which demonstrates the importance of salicylic acid for LAR and SAR(Metraux, 2010, p. 1). The discovery of the importance of salicylic acid in providing disease resistance has lead researchers to study its biosynthesis. It has been recognized that salicylic acid is derived from the shikimatephenylpropanoid pathway. Two routes have been developed that differ at the step involving the hydroxylation of the aromatic ring. Regardless, the starting reactant is the same, phenylalanine – one of the twenty common amino acids. It is a non-polar, Salicylic Acid 7 electrically neutral amino acid that biochemically forms proteins that are coded by DNA. The mechanism of synthesis starts with phenylalanine is converted to cinnamic acid by the enzyme phenylalanine ammonia lyase. Cinnamic acid subsequently becomes hydroxylated to produce the ortho-coumaric acid followed by the oxidation of the side chain. Alternatively, the side chain of cinnamic acid is initially oxidized to give benzoic acid, which is followed by the hydroxylation in the ortho position (Metraux, 2010, p. 1). Initially, salicylic acid was suggested to be synthesized without benzoic acid; however, later results showed that benzoyl glucose, a conjugated form of benzoic acid, was most probable to be the precursor of salicylic acid. The cinnamic acid-derived synthesis of salicylic also occurs in rice, cucumbers, and potatoes. The shikimate-phenylpropanoid pathway and its derivatives are outlined in Figure 4. Salicylic Acid Figure 4: The biosynthetic pathways for salicylic acid biosynthesis. The part highlighted in blue was originally described in bacteria and has now been shown to take place in the chloroplast of plants. The ICS enzyme is isochorismate synthase. (Metraux, 2010) 8 Salicylic Acid 9 Conversely, not all aspects of salicylic acids are as beneficial as providing defense for plants and anti-inflammatory agents for humans. Salicylic acid frequently serves as a pollutant in multiple industrial wastewaters. To combat the contamination through a salicylic acid means, uncatalyzed wet air oxidation has recently been proposed. It is considered a hopeful technique for treating these phenolic effluents (Collado, Garrido, Laca, & Diaz, 2010, p. 8629). The role of the oxygen in the wet oxidation mechanism takes place in the radical initiation process. An increase in the amount of radicals that are generated as well as an increase in the oxidation rate is implied by a higher concentration of dissolved oxygen (Collado, Garrido, Laca, & Diaz, 2010). The radical initiation in the first step occurs as a result of the high operating pressure. The overall steps of the radical initiation are seen in reactions 1-5 below: Figure 5 (Collado, Garrido, Laca, & Diaz, 2010) Salicylic Acid 10 The proposed reaction pathway starts with salicylic acid undergoing decarboxylation to phenol. Subsequently, the degradation of phenol to dihydroxylated rings (catechol and hydroquinone) occurs. (Collado, Garrido, Laca, & Diaz, 2010). Reportedly, hydroquinone as an intermediate is presented in mutiple oxidations of phenolic compouds. However when tested with researchers Collado, Garrido, Laca and Diaz, it could not be confirmed due to high instability. The aromatic intermediates created colorful compounds such as o-benzoquinone and pbenzoquinone – whose appearance eventually went on to confirm the formation of hydroquinone. The proposed reaction mechanism is outlined in the figure below: Salicylic Acid 11 Figure 6: Proposed reaction mechanism for the noncatalytic wet oxidation of salicylic acid. (Collado, Garrido, Laca, & Diaz, 2010) 12 Salicylic Acid References 1. Collado, S., Garrido, L., Laca, A., & Diaz, M. (2010). Wed Oxidation of Salicylic Acid Solutions. Environmental Science Technology , 8629-8635. 2. Metraux, J.-P. (2010). Recent breakthroughs in the study of salicylic acid biosynthesis. TRENDS in Plant Science , 1-3. 3. Shalmashi, A., & Eliassi, A. (2008). Solubility of Salicylic Acid in Water, Ethanol, Carbon Tetrachloride, Ethyl Acetate, and Xylene. Journal of Chemical and Engineering , 53 (1), 199-200. 4. Taber, D. F. (2011, October 17). Organic Chemistry Highlights. Retrieved 4 18, 2014, from Organic Chemistry Portal: http://www.organicchemistry.org/Highlights/2011/17October.shtm 5. Williamson, K. L. (1994). Synthesis of Acetylsalicylic Acid (Aspirin). In K. L. Williamson, Macroscale and Microscale Organic Experiments (Vol. 2, pp. 379382). Boston: Houghton Mifflin.