Barbara Melosky, MD, FRCPC
advertisement

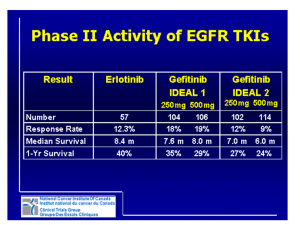
Challenges in Designing and Conducting Clinical Trials in Oncology Barbara Melosky, MD, FRCPC Objectives • Provide an overview of key issues and challenges in designing and conducting oncology clinical trials • Discuss regional and country-specific issues • Highlight issues in the era of personalised medicine What Is a Clinical Trial? • Mechanism to learn how to improve treatments • Mechanism to introduce new treatment techniques (procedures) or new drugs • Method to build on prior knowledge of technique or drug What Is NOT a Clinical Trial? • Random experiments by devious physicians looking to make money or become famous • Crazed make-work project for nurses and data collectors • A way to avoid animal rights activists • Conspiracy to get drugs/products into the market HOW TO OPEN A TRIAL: The Long Journey PI is approached about a potential trial 1. 2. 3. 4. 5. 6. ISSUES TO CONSIDER Medical need Costs per patient Can it accrue? Workload implications Strategic value Academic value TRIAL APPROVED REB Resource Impact Analysis CONTRACT WAITLIST Key Components of a Clinical Trial • Identification of a relevant clinical question – Better treatment or schedule? • An answerable question – Statistics (numbers, power of the intervention, results expected) • Realistic intervention or treatment – Plan for the future • Discussed by peer experts and ethics boards Type 1 Error (Alpha) • Probability of rejecting the null hypothesis when it is in fact true (i.e. no difference) • Not as big a concern for early phase (exploratory studies) • More of an issue for phase III trials Type II Error (Beta) • Probability of accepting null hypothesis when it is false • Power = 1-Beta • Don’t want to do this in early phase studies or will not proceed further Sample Size Calculation • Based on 3 factors: – Delta (expected difference between arms) – Alpha – Beta • Need to consider: – How many patients can be reasonably accrued – How much time – How much money Online Sample Size Calculators • Simon 2-stage: http://www.cscc.unc.edu/cscc/aivanova/SimonsTwoStageDesign.aspx • Traditional design: http://www.stat.ubc.ca/~rollin/stats/ssize/b2.html Phases of Trials • Phase I: Looks at side effects and doses – Any suggestion of particular type of cancer that might be more sensitive • Phase II: Looks for a suggestion of efficacy in a specific cancer (e.g. lung cancer) – Usually about 75 people per study • Phase III: Is this new treatment better than the standard treatment? – Often randomised or with a placebo arm Phase I • Assess tolerance of drug/device • Find maximum tolerated dose (MTD)/recommended phase II dose (RP2D) • Generally small sizes – Standard 3+3 design Standard Phase I Design Phase I • Pharmacokinetics (PK) • Pharmacodynamics (PD) • Preliminary efficacy – Phase 1b Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer Julie R. Brahmer, M.D., Scott S. Tykodi, M.D., Ph.D., Laura Q.M. Chow, M.D., Wen-Jen Hwu, M.D., Ph.D., Suzanne L. Topalian, M.D., Patrick Hwu, M.D., Charles G. Drake, M.D., Ph.D., Luis H. Camacho, M.D., M.P.H., John Kauh, M.D., Kunle Odunsi, M.D., Ph.D., Henry C. Pitot, M.D., Omid Hamid, M.D., Shailender Bhatia, M.D., Renato Martins, M.D., M.P.H., Keith Eaton, M.D., Ph.D., Shuming Chen, Ph.D., Theresa M. Salay, M.S., Suresh Alaparthy, Ph.D., Joseph F. Grosso, Ph.D., Alan J. Korman, Ph.D., Susan M. Parker, Ph.D., Shruti Agrawal, Ph.D., Stacie M. Goldberg, M.D., Drew M. Pardoll, M.D., Ph.D., Ashok Gupta, M.D., Ph.D., and Jon M. Wigginton, M.D. Abstract BACKGROUND: Programmed death 1 (PD-1) protein, a T-cell coinhibitory receptor, and one of its ligands, PD-L1, play a pivotal role in the ability of tumor cells to evade the host's immune system. Blockade of interactions between PD-1 and PD-L1 enhances immune function in vitro and mediates antitumor activity in preclinical models. METHODS: In this multicenter phase 1 trial, we administered intravenous anti-PD-L1 antibody (at escalating doses ranging from 0.3 to 10 mg per kilogram of body weight) to patients with selected advanced cancers. Anti-PD-L1 antibody was administered every 14 days in 6-week cycles for up to 16 cycles or until the patient had a complete response or confirmed disease progression. RESULTS: As of February 24, 2012, a total of 207 patients--75 with non-small-cell lung cancer, 55 with melanoma, 18 with colorectal cancer, 17 with renal-cell cancer, 17 with ovarian cancer, 14 with pancreatic cancer, 7 with gastric cancer, and 4 with breast cancer--had received anti-PD-L1 antibody. The median duration of therapy was 12 weeks (range, 2 to 111). Grade 3 or 4 toxic effects that investigators considered to be related to treatment occurred in 9% of patients. Among patients with a response that could be evaluated, an objective response (a complete or partial response) was observed in 9 of 52 patients with melanoma, 2 of 17 with renal-cell cancer, 5 of 49 with non-small-cell lung cancer, and 1 of 17 with ovarian cancer. Responses lasted for 1 year or more in 8 of 16 patients with at least 1 year of follow-up. CONCLUSIONS: Antibody-mediated blockade of PD-L1 induced durable tumor regression (objective response rate of 6 to 17%) and prolonged stabilization of disease (rates of 12 to 41% at 24 weeks) in patients with advanced cancers, including non-small-cell lung cancer, melanoma, and renal-cell cancer. (Funded by Bristol-Myers Squibb and others; ClinicalTrials.gov number, NCT00729664.). Brahmer JR, et al. N Engl J Med. 2012 Jun 28;366(26):2455-65. Epub 2012 Jun 2. SAFETY A maximum tolerated dose was not reached. The median duration of therapy was 12 weeks (range, 2 to 111 weeks) (Table S2A in the Supplementary Appendix). A relative dose intensity of at least 90% was achieved in 86% of patients. Of the 207 patients, 23 (11%) discontinued treatment because of an adverse event; of these events, 12 (6%) were considered by investigators to be related to treatment (Tables S2B and S3A in the Supplementary Appendix). PHARMACOKINETICS AND PHARMACODYNAMICS Serum levels of anti–PD-L1 antibody increased in a dose-dependent manner from 1 to 10 mg per kilogram in 131 patients who were evaluated. The geometric mean area under the curve (0 to 14 days) for doses of 1 mg, 3 mg, and 10 mg per kilogram were 2210, 7750, and 36,620 µg per milliliter per hour, respectively (coefficient of variation, 34 to 59%). After the first dose, geometric mean peak levels at these dose levels were 27, 83, and 272 µg per milliliter, respectively (coefficient of variation, 30 to 34%). The half-life of anti–PD-L1 antibody was estimated from population pharmacokinetics as approximately 15 days. PDL1 receptor occupancy on CD3+ peripheral-blood mononuclear cells was assessed in 29 patients with melanoma at the end of one cycle of treatment, at doses of 1 to 10 mg per kilogram. Median receptor occupancy exceeded 65% for all groups (Fig. S2 in the Supplementary Appendix). Brahmer JR, et al. N Engl J Med. 2012 Jun 28;366(26):2455-65. Epub 2012 Jun 2. Table 1. Adverse Events of Special Interest in 207 Patients Receiving Anti-PD-L1 Antibody Anti-PD-L1, 0.3 mg/kg (N=3) Event All Grades Grade 3 or 4 Anti-PD-L1, 1 mg/kg (N=37) All Grades Grade 3 or 4 Anti-PD-L1, 3 mg/kg (N=42) All Grades Grade 3 or 4 Anti-PD-L1, 10 mg/kg (N=125) Anti-PD-L1, Total (N=207) All Grades Grade 3 or 4 All Grades Grade 3 or 4 Number of patients (%) Any adverse event of special interest†† 1 (33) 0 18 (49) 2 (5) 14 (33) 2 (5) 48 (38) 6 (5) 81 (39) 10 (5) Any rash 0 0 5 (14) 0 1 (2) 0 8 (6) 0 14 (7) 0 Pruritus 0 0 6 (16) 0 3 (7) 0 3 (2) 0 12 (6) 0 Vitiligo 0 0 3 (8) 0 1 (2) 0 1 (1) 0 5 (2) 0 Pruritic rash 0 0 1 (3) 0 1 (2) 0 2 (2) 0 4 (2) 0 Macular rash 0 0 2 (5) 0 1 (2) 0 0 0 3 (1) 0 Erythema 0 0 2 (5) 0 0 0 0 0 2 (1) 0 Erythematous rash 0 0 0 0 1 (2) 0 1 (1) 0 2 (1) 0 1 (33) 0 4 (11) 0 6 (14) 0 8 (6) 0 19 (9) 0 0 0 0 0 2 (5) 0 19 (15) 1 (1) 21 (10) 1 (<1) Hypothyroidism 0 0 0 0 1 (2) 0 5 (4) 0 6 (3) 0 Adrenal insufficiency 0 0 0 0 1 (2) 1 (2) 1 (1) 0 2 (1) 1 (<1) Autoimmune thyroiditis 0 0 2 (5) 0 0 0 0 0 2 (1) 0 0 0 0 0 2 (5) 0 0 0 2 (1) 0 0 0 1 (3) 0 0 0 2 (2) 0 3 (1) 0 Skin or subcutaneous disorder Gastrointestinal disorder Diarrhea Procedural complication Infusion-related reaction Endocrine disorder Eye disorder Dry eye Immune-system disorder Hypersensitivity Laboratory investigation †The numbers reported within a column may not add up to the total number reported because patients who had more than one adverse event were counted for each event but were counted only once for “any adverse events of special interest.” Brahmer JR, et al. N Engl J Med. 2012 Jun 28;366(26):2455-65. Epub 2012 Jun 2. Table 2 Clinical Activity of Anti–PD-L1 Antibody in the Efficacy Population.* Tumor Type and Dose No. of patients Objective Response Duration of Response Stable Disease ≥24 Weeks Rate of Progression-free Survival at 24 Weeks No. of patients % (95% CI) Mo No. of patients % (95% CI) % (95% CI) Melanoma 0.3 mg/kg 1 0 0 (0–98) NA 0 0 (0–98) NA 1 mg/kg 18 1 6 (0–27) 6.9 6 33 (13–59) 39 (16–61) 3 mg/kg 17 5¶ 29 (10–56) 23.5+, 22.9+, 16.2+, 4.1+, 3.5 3 18 (4–43) 47 (21–72) 10 mg/kg 16 3‖ 19 (4–46) 20.8+, 16.6, 2.8 5 31 (11–59) 44 (19–68) All doses 52 9 17 (8–30) 14 27 (16–41) 42 (28–56) All patients, 1 mg/kg 11 0 0 (0–29) NA 0 0 (0–29) NA All patients, 3 mg/kg 13 1 8 (0–36) 2.3+ 1 8 (0–36) 34 (7–60) Squamous subtype 4 0 0 (0–60) NA 1 25 (0–81) 50 (1–99) Nonsquamous subtype 9 1 11 (0–48) ND 0 0 (0–34) 25 (0–55) 25 4 16 (5–36) 16.6+, 12.6+, 9.8, 3.5 5 20 (7–41) 46 (25–67) Squamous subtype 8 1 13 (0–53) ND 2 25 (3–65) 47 (10–83) Nonsquamous subtype 17 3 18 (4–43) ND 3 18 (4–43) 46 (20–72) 49 5 10 (3–22) 6 12 (5–25) 31 (17–45) Squamous subtype 13 1 8 (0–36) ND 3 23 (5–54) 43 (15–71) Nonsquamous subtype 36 4 11 (3–26) ND 3 8 (2–23) 26 (10–42) 1 0 0 (0–98) NA 0 0 (0–98) NA 3 mg/kg 1 0 0 (0–98) NA 0 0 (0–98) NA 10 mg/kg 16 1 6 (0–30) 1.3+ 3 19 (4–46) 25 (4–46) All doses 17 1 6 (0–29) 3 18 (4–43) 22 (2–43) 17 2 12 (2–36) 7 41 (18–67) 53 (29–77) Non–small-cell lung cancer All patients, 10 mg/kg All patients, all doses Ovarian cancer Renal-cell cancer, 10 mg/kg 17, 4 * The efficacy population included 160 patients in whom a response could be evaluated and who initiated treatment by August 1, 2011. These patients had measurable disease at a baseline tumor assessment and at least one of the following: an assessment of tumor burden during the study, clinical progression, or death. NA denotes not applicable, and ND not determined. † Objective response rates (including both complete response and partial response) are based on confirmed responses only, with 95% confidence intervals calculated with the use of the Clopper–Pearson method. ‡ The duration of response is the time from the first response to the time of documented disease progression, death, censoring of data (denoted by a plus sign), or last tumor assessment. § The rate of progression-free survival was the proportion of patients who did not have disease progression and were alive at 24 weeks, as calculated by the Kaplan–Meier method. The Greenwood method was used to calculate confidence intervals. ¶ Two of these patients had a complete response. ‖One of these patients had a complete response. Phase II • Performed in patients with same cancer • Assess efficacy • Can be single arm or randomised – Interpret results with caution! – The MetMab Saga Onartuzumab: A One-armed (Monovalent) anti-MET Antibody Ridgway JBB, et al. Protein Eng 1996; Nguyen TH, et al. Cancer Gene Ther 2003; Kong-Beltran M, et al. Cancer Cell 2004; Martens T, et al. Clin Cancer Res 2006; Merchant M, et al. Proc Natl Acad Sci USA 2013. Courtesy of Barbara Melosky, MD. Phase II: Erlotinib ± Onartuzumab 2nd/3rd-line NSCLC 1:1 Key eligibility: • Stage IIIB/IV • 2nd/3rd-line • Tissue • PS 0–2 n=137 Spigel DR et al. J Clin Oncol. 2013;31:4105-14. R A N D O M I S A T I O N Arm A n=68 Placebo + erlotinib Onartuzumab Arm B n=69 + erlotinib Efficacy in Met Diagnostic Positive Patients OS: HR = 0.37, p = 0.002 PFS: HR = 0.53, p = 0.04 MetMAb + erlotinib 2.9 Median (mo) 1.0 1.0 0.8 0.8 Probability of Survival Probability of Progression Free Median (mo) Placebo + erlotinib 1.5 0.6 0.4 0.2 0.0 Placebo + erlotinib 3.8 MetMAb + erlotinib 12.6 0.6 0.4 0.2 0.0 0 3 6 9 12 15 Time to Progression (months) Spigel DR et al. J Clin Oncol. 2013;31:4105-14. 18 0 3 6 9 12 15 18 Overall Survival (months) 21 ASCO 2014 PHASE lll OAM4971g: Overall Survival Results NEGATIVE TRIAL Courtesy of Barbara Melosky, MD. Phase III • Larger samples (~60-5000), multicentre • Control group (placebo or active control) • Randomisation • Safety and QOL BR.21 Trial Endpoints Patients • Stage IIIB/IV • NSCLC with PS 0–3 • Previously treated Erlotinib 150 mg daily 2:1 randomisation Placebo Shepherd et al. N Engl J Med. 2005;353:123-132. Primary • Overall survival (OS) Secondary • Progression-free survival (PFS) • Response rate (RR) • Safety • Quality of life (QoL) • Duration of response BR.21: Overall Survival 1.00 Erlotinib (n=488) Placebo (n=243) Median Survival (months) 6.7 4.7 1-year survival (%) 31 21 Survival distribution function Endpoint HR = 0.70 (95% CI, 0.58-0.85); P<0.001 0.75 0.50 31% 42.5% improvement in median survival 0.25 Erlotinib Placebo 21% 0 0 5 10 15 Survival time (months) Shepherd et al. N Engl J Med. 2005;353:123-132. 20 25 30 Phase IV • Studies performed to assess long-term side effects/efficacy • Open labeled • May be a way to give access to new therapies ENDPOINTS DFS • Time from randomisation until objective tumour recurrence or death • Accepted by FDA as a primary endpoint for drug approval • Useful in adjuvant trials: long-term follow-up – Catches long-term toxicity and second malignancies PFS • Time from randomisation until objective tumour progression or death • Accepted by FDA as a primary endpoint for drug approval – Especially with quality of life • Definition of tumour progression should be carefully detailed in the protocol IPASS: First-line Study Design Endpoints Patients Primary • Chemo-naive • Age ≥18 years • Adenocarcinoma histology • Never or light exsmokers • Life expectancy ≥12 weeks • PS 0-2 • Measurable stage IIIB/ IV disease Gefitinib (250 mg/day) • PFS (non-inferiority) Secondary 1:1 randomisation Carboplatin (AUC 5 or 6)/ paclitaxel (200 mg/m2) 3 weekly • ORR • OS • QoL • Disease-related symptoms • Safety and tolerability Exploratory • Biomarkers • EGFR mutation • EGFR gene copy number • EGFR protein expression Mok et al 2009 Courtesy of Barbara Melosky, MD. Courtesy of Barbara Melosky, MD. TTP • Time from randomisation until objective tumour progression (RECIST) • TTP does not include deaths • Accepted by FDA as a primary endpoint for drug approval TTF • Time from randomisation to discontinuation of treatment for any reason – Disease progression, death and treatment toxicity – Treatment toxicity • Patient or physician withdrawal, or patient intolerance – TTF is not recommended as a regulatory endpoint for drug approval OS • Time from randomisation to death for any reason • Gold endpoint • No bias • DEAD is DEAD PFS VS OS ASCO 2008: FLEX FLEX Study Design NSCLC wet IIIB/IV EGFRexpressing Chemotherapy + Cetuximab Chemotherapy Primary Endpoints OS Chemotherapy (CT) Cetuximab Cisplatin 80 mg/m2 day 1 Initial dose 400 mg/m2 Vinorelbine 25 (30) mg/m2 days 1, 8 Then 250 mg/m2 weekly Every 3 weeks, up to 6 cycles Pirker et al. Lancet. 2009;373:1525-1531. Maintenance Cetuximab until PD or intolerable toxicity Flex Results 100 Overall Survival (%) 90 80 Median OS 1-Y OS CV + Cetuximab 11.3 mo 47% CV 10.1 mo 42% 70 HR: 0.871; P=0.044 60 50 40 30 20 10 0 0 6 12 18 24 30 Months CV + Cetuximab CV P-value RR 36% 29% .012 PFS 4.8 mo 4.8 mo NS TTF 4.2 mo 3.7 mo .015 Pirker R et al. J Clin Oncol. 2008;26:3. First-line Bevacizumab in NSCLC: Two Positive Phase III Trials E45991 Trial commenced 2001 Endpoint CP x 6 (n=444) Stage IIIB/IV non-sq. NSCLC (n=878) OS Bevacizumab (15 mg/kg) q3w + CP x 6 (n=434) AVAiL2 Trial commenced 2005 Stage IIIB/IV non-sq. NSCLC (n=1,043) PD* 2 R A N D O M I S E 1 1 2 Bevacizumab (15 mg/kg) q3w + CG x 6 (n=351) Bevacizumab PD Bevacizumab PD Placebo + CG x 6 (n=347) PD* Placebo + CG x 6 Bevacizumab (7.5 mg/kg) q3w + CG x 6 (n=345) *No crossover permitted. CG = cisplatin/gemcitabine 1. Sandler et al. N Engl J Med. 2006; 2. Reck et al. J Clin Oncol. 2009. Bevacizumab PD PFS E4599 Overall Survival 12 mo 24 mo RR PC 44% 15% 15% PCB 51% 23% 35% 1.0 Probability 0.8 HR: 0.79 (0.67, 0.92) P = .003 0.6 Medians: 10.3, 12.3 0.4 0.2 0 0 6 12 18 Months Sandler et al. N Engl J Med. 2006;355:2542-2550. 24 30 36 AVAiL • Original endpoint OS • When E4599 came out showing improved PFS and OS, endpoint in AVAiL changed to PFS • Follow-up of patients q3 months did not change Reck M et al. J Clin Oncol. 2009;27:1227-34. Progression-free Survival 1.0 Probability of PFS Placebo + CG 0.8 HR (95% CI) p value 0.6 Median PFS (months) 6.2 Bev 7.5 mg/kg + CG Bev 15 mg/kg + CG 0.75 (0.64–0.87) 0.85 (0.73–1.00) 0.0003 0.0456 6.8 6.6 0.4 0.2 0 0 6 12 18 24 30 3 5 0 0 0 0 Time (months) No. at risk Placebo + CG Bev 7.5 mg/kg + CG Bev 15 mg/kg + CG 2008 IASLC/ESMO update. 347 345 351 178 214 200 34 63 57 12 18 12 AVAiL: No Significant Benefit in OS Placebo + CG 1.0 HR (95% CI) Probability of OS 0.8 p value Median PFS (months) 13.1 Bev 7.5 mg/kg + CG Bev 15 mg/kg + CG 0.93 (0.78–1.11) 1.03 (0.86–1.23) 0.42 0.76 13.6 13.4 0.6 0.4 0.2 0 0 6 12 18 Time (months) ESMO 2008. 24 30 36 AVAiL: High Proportion of Patients Received Post-Protocol Therapies Post-protocol therapy Placebo + CG % (n) Bev 7.5 mg/kg + CG % (n) Bev 15 mg/kg + CG % (n) Any treatment, % (n) 65 (224) 61 (210) 61 (214) TKIs 41 (92) 48 (100) 42 (90) Chemotherapy 73 (164) 65 (137) 69 (147) Angiogenesis inhibitors <1 (2) <1 (2) <1 (3) Surgical and medical procedures 26 (60) 34 (71) 27 (57) Type, % (n) AVAiL: Trend Towards Improved OS in Bevacizumab-Treated Patients Not Receiving Post-Protocol Therapies 1.0 Median OS (95% CI) Bev No 2nd-line Bev 8.7 (7.8-9.9) Placebo 7.3 (5.9-8.9) Probability of OS HR (p value) 0.8 Bev to placebo 0.84 (0.20) 0.6 0.4 0.2 Bev without 2nd-line Placebo without 2nd-line n=123 n=272 0 10 20 Time (months) 30 40 Country-specific Issues • US represents 50% of global market • Pharma will ensure US representation on trials • The remainder will come from: – China – India – Eastern Europe – Latin America – Europe – Canada Clinical Trial Trends • More global, more competitive environment • Larger trials: – 6 vs 3 months of FOLFOX stage III colon, N=10,000 • Tissue required for colorectal, breast, lymphoma… Tissue Collection Challenges • Tissue collection vital: eligibility, correlative studies • Pathology departments reluctant to send blocks: – REB of other institutions not acknowledged – Concern about exhausting archival tissue – Costs of processing and shipping Biggest Trend in Clinical Trials • Biomarker-driven! • Narrower eligibility – cancer subsets: – Bevacizumab in triple-negative breast cancer – KRAS wild type in colorectal (60%)…RAS wild type in colorectal (45%)…future (30%) – ALK NSCLC (4%) – ROS NSCLC (1%) PD-L1 Biomarker Short History • Feb 2008: Roche acquires Ventana Medical Systems – PD-L1 IHC (MPDL3280A) • Dec 2011: BMS collaborates with Dako for the development of pharmacodiagnostic predictive tests (including PD-L1) • May 2014: Merck collaborates with Dako to develop a companion diagnostic test for the analysis of tumour PD-L1 • June 2014: MedImmune/AZ MEDI4736 announces agreement with Ventana to develop PD-L1 test INVESTIGATOR-INITIATED TRIALS Treatment of rash secondary to erlotinib PANCANADIAN RASH TRIAL WITH EGFR INHIBITORS Barbara Melosky, Helen Anderson, Ron Burkes, Quincy Chu, Desiree Hao, Vincent Ho, Cheryl Ho, Wendy Lam, Christopher Lee, Natasha Leighl, Nevin Murray, Sophie Sun, Robert Winston, Janessa Laskin Corresponding author: bmelosky@bccancer.bc.ca Erlotinib-induced Rash • Affects 50% – 75% of patients • Most often appears on the face and chest • May be severe • May limit exposure to erlotinib, compromising efficacy Study Design Incidence of Rash and Time to Severe Grade 3 Rash Incidence of any rash n (%) ARM 1 (N=50) 84% ARM 2 (N=50) 84% Arm 3 (N=50) 82% Incidence of Grade 3 rash n (%) P value Mean (days) to Grade 3 rash onset P value 9.5% P= 0.034 Arm 1 vs 3 17.4 P = 0.0147 14.3% P= 0.065 Arm 2 vs 3 13.3 34.1% 12 Overall Survival Not significant but Arm 3 does the worst Conclusions ASCO 2014 • Prophylactic minocycline reduces the incidence of severe EGFR inhibitor– induced rash without influencing efficacy • Prophylactic minocycline should be considered upon initiation of EGFR inhibitors • Rash is a predictive factor of survival and should be treated Investigator-Initiated Trial • My idea • PanCanadian trial • Randomised prospective • Took 5 years! (first 2 were contract) • Budget 500,000…900,000 • End of day: Completed it, presented it at ASCO and publishing it Who Benefits From Clinical Research? … or who doesn’t? RESEARCHERS TAKE BETTER CARE OF PATIENTS RESEARCH SAVES MONEY RESEARCH DRIVES CME RESEARCH SAVES LIVES RESEARCH BRINGS GRANTS PATIENT ACCESS TO NEW AGENTS STATE-OF-THE-ART FACILITIES JOB CREATION RECRUIT THE BEST BY BEING THE BEST Who Benefits? The Patient! IT’S COMPLICATED ! Courtesy of Barbara Melosky, MD.