Temperature, Heat, and Expansion
advertisement

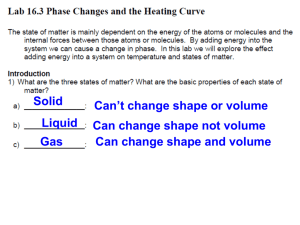
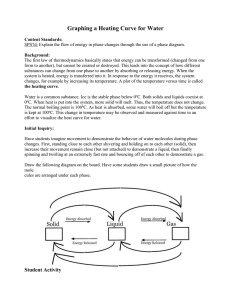
Temperature, Heat and Expansion Jillian Campbell, Karly Johnson, Jared Ostler, Daniel Borbolla Temperature • • • • • • Temperature is the quantity that indicates how warm or cold an object is with respect to some standard. The thermometer measures temperature and it was invented in 1602 by Galileo. Nearly all materials expand when their temperature is raised and contract when their temperature is lowered. It is measured in degrees. The International scale is 0 is the temperature at which water freezes and 100 is the temperature at which water boils. The space between is divided into 100 equal parts called degrees; hence this thermometer is called a centigrade thermometer (from centi, “hundredth,” and gradus, “step”). In the United States the number 32 is the temperature in which water freezes and 212 is the temperature at which water boils. This is a Fahrenheit thermometer. Heat • • • • • • • Heat is the energy transferred from one object to another because of a temperature difference between them. The direction of spontaneous energy transfer is always from a warmer object to a neighboring cooler object. Heat is measured in Joules. Matter doesn’t contain heat, matter contains molecular energy and possibly potential energy, not heat. Energy from heat flow is internal energy which is the grand total of all energies inside a substance. Just as dark is the absence of light, cold is the absence of thermal energy. For example, a barrelful of hot water will transfer more heat to a cooler substance than a cupful of water will. There is more internal energy in the larger volume of water. Difference between temperature and heat • • • Heat: The energy transferred from one object to another because of a temperature difference between them. Temperature: The quantity that indicates how warm or how cold an object is with respect to some standard. The main difference between heat and temperature is that without heat or cold (absence of heat), there is no temperature. Temperature is simply a way of measuring how much of heat is present. For example you have a loaf of bread right out of the oven, if you touch it you will get burnt but you can’t really tell just how hot it really is. If you stick a thermometer in it, you will be able to see just how much heat it is still conducting. If stick a thermometer into the same loaf of bread after an hour you will see that the temperature will be much lower. This does not mean that there is no heat in it, but that the heat has been reduced so much that you cannot really feel it. Specific heat capacity • • • • • • • Specific heat capacity can be defined as the heat required to raise the unit mass of a substance by one degree of temperature. The specific heat capacity of any substance can also be defined as the quantity of heat required to change the temperature of a unit mass of the substance by one degree. This means that when you are heating a substance, the specific heat capacity is the amount of heat that would need to be applied in order to increase the temperature of the substance by just one degree. It doesn’t need to be a significant change in temperature. Only one degree of change in temperature is required. Delta Q = mc delta T where, : Q= Heat supplied to substance, m= Mass of the substance, c= Specific heat capacity, T= Temperature rise. Expansion • • • • Expansion is the tendency of matter to change in volume in response to a change in temperature. When the temperature of a substance is increased, its molecules or atoms jiggle faster and move farther apart, on the average. The result is an expansion of the substance. This means that when a substance is heated, the molecules contract making the substance expand. We have to account for expansion in structures that we make. For example, when railroads are made, they leave a gap between the rails to accommodate for the expansion of the rails when it gets hot outside. The same idea is used when making sidewalks. We put gaps between the pieces of sidewalk because of expansion. Expansion of Water • • • • • Water is like most other substances in that it’s molecules expand when heated. However, water doesn’t expand in the temperature range between 0° C and 4° C. The reason this happens is because ice has a crystalline structure, with open structured crystals. Water molecules in this open structure occupy a greater volume than they do in the liquid phase. This means that ice is less dense than water. However, when ice begins to melt, not all of the open-structured crystals collapse. Some crystals remain in the ice-water mixture, making “slush” that makes the water somewhat “bloated”, slightly increasing the volume. This process is called expansion and results in ice water being less dense than slightly warmer water. Water undergoes these two processes of contraction and expansion simultaneously. The collapsing effect dominates until water’s temperature reaches 4° C, after which expansion overrides contraction because most of the microscopic ice crystals have melted by then. References • Text book • http://hyperphysics.phyastr.gsu.edu/hbase/thermo/temper.html • http://hyperphysics.phyastr.gsu.edu/hbase/thermo/heat.html