Eukaryotic Gene Regulation
advertisement

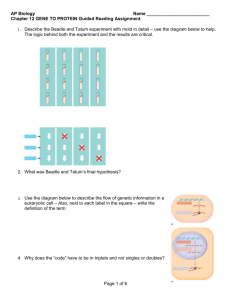
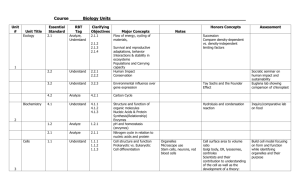
Regulation of eukaryotic gene sequence expression Lecture 6 Introduction • Difference between eukaryotic and prokaryotic DNA • Brief Overview of Eukaryotic “gene” expression regulation. • Regulation at the transcription level • Post transcriptional regulation – Alternative Splicing • Pre/ Post-translation modification Prokaryotic DNA & Eukaryotic DNA • The following gives the broad differences between the different cell classes: 1. Prokarytoic is circular, eukaryotic is straight. 2. Eukarytoic DNA is in the form of chromatin [prokaryotic ]; essentially Eukaryotic is surrounded by a histone envelope. 3. Prokaryotic genes are in the form of operons[ with polycistronic, multiple genes, mRNA]; e.g. lac operon, while eukaryotic DNA is monocistronic, one gene. Prokaryotic DNA & Eukaryotic DNA 4. The promoter regulatory systems in prokaryotic has a repressor gene sequence; while in eukaryotic DNA there are enhancers and silencers sequences. 5. The protein coding region of Eukaryotic DNA consists of exons [ regions that are translated] interjected with introns [regions that are not translated]. In prokaryotic DNA the sequences is continuous [ no introns] Overview of Eukaryotic gene regulation • Eukaryotic cells have more than one level of “transcription” control. • The students can refer to the supplementary lecture for details on: – Chromatin remodelling (level 1) – miRNA (level 5) level 2: Expression Ctrl at the transcription level • More complex than prokaryotic consists of: • Promoter: like prokaryotics is the region where RNA polymerase binds. [ refer to p region in the lac operon] – There different promoter “regulatory” sites : e.g. core (basal promoter), distal (upstream )promoter. • Enhancers: regions that increase transcription levels • Silencers: regions that decrease the level of transcription • Both enhancers and silencers can be thousands of bp away form the transcription site • Similar to prokaryotic cells promoters have specific sequences [refer to supplementary notes] level 2: An eukaryotic promoter sequence The Core promoters regions : Just upstream of where RNA polymerase binds and transcription starts [transcription start site] Contains TATA and/or CAAT boxes and/or CG rich level 2: Expression Ctrl at the transcription level • Enhances: – DNA sequences that can be located: upstream or downstream of the “gene” – Required to achieve maximum level of expression – They seem to be generic to an extent (an enhancer need not be gene specific ([1] p 322) – They can also be inside the gene they regulate; Ig heavy chain enhancer. – Can enhance more than one gene; e.g. β and ε globins in chickens (ref [1] p. 322) – There activation is time and tissue specific (play a part in organism development.) level 2: Expression Ctrl at the transcription level • Silencers : – Repress the level of transcription that was initiated by the corresponding promoter. – Their location like enhancers can be in different locations. – Are tissue specific and time-specific – The following example illustrates how silencers and enhancers work in tandem to control gene expression: • E.g. found in gene that produces a hormone involved in thyroid production/stimulation . This hormone is only produced in pituitary cells. Expression only occurs in these cells because of a silencer that binds a cellular factor which repress transcription. However, in cells that are required to produce the hormone the effect of the silencer is itself neutralised by an enhancer located 1.2 kb upstream of the promoter of the gene and is only “activated” in the cells [thyrotrophs] that must produce this hormone Level 3: Exon and intron sequences • The coding region [gene] of Eukaryotic DNA consists of regions called exons interjected with introns. • Prior to translation these introns must be “cut out” spliced from the pre mRNA [ mRNA] to produce mature mRNA • The following figure shows the DNA sequence of a eukaryotic gene “system” (in will be discussed in more detail in the lecture: “Finding genes” Level 3: Alternative splicing • In 40% to 60% of genes the introns can be spliced in a number of ways to produces alternative spliced mature mRNA strands. Only mature mRNA strands are translated into amino acid strands. • The consequence of this process [Alternative Splicing] is that one DNA coding region can produce many mature mRNA strands and so many proteins. With some genes being able to produce ~38,000 different mature mRNA strands [splices]. Mostly associated with immunological proteins. • Like DNA transcription RNA splicing is also regulated (refer to supplementary notes.) • Methods and computational techniques used to detect splices can be found in Alternative splicing: global insights [not required reading! But may prove interesting] Level 3: Basic Alternative splicing Adapted from [3] • The above illustrate what is referred to as (mutually exclusive) splicing. • There are four possible types of splicing. Types of Alternative splicing A more comprehensive description A.S. can be found at ref [5 and 6] Alternative splicing: the effects • Alternative splicing can lead to: 1. use of a different site for translation initiation (alternative initiation); alternative promoter/exon. What might this mean in relation to the DNA sequence? What does it suggest about the possible location of promoters? 1. a different translation termination site by the addition/removal of a stop codon in the coding sequence (alternative termination). 3. Alternative splicing can also change the internal region because of an in-frame insertion or deletion. Level 4: The CAP and Poly A tail • After splicing the mRNA undergoes two other changes. These can be used to distinguish pre-mRNA form mature mRNA • • The poly A tail, polyadenylation, is a sequence of adenine (A) RNA molecules added to the end (3’ end) of the mature mRNA. In addition a “CAP” complex [modified G RNA molecule] is added to the 5’ start of the mature mRNA; • both play a part in protecting the mRNA from degradation while it is being transported into the cytoplasm and to the ribosomes. • Level 5 (Degradation of mRNA by microRNA strands) and level 6 and 7 (translational and post translational) can be looked at in supplementary material Level 6: Translation/post translational Modification • The protein levels and activities can also be controlled via: – Regulation of the protein stability and modification – An example of such a protein is p53 a transcription factor for a number of important cell cycle genes. – Normally its low but in a damaged cell levels increase and helps express these important genes – When cells are damaged P53 Protein modification results in its activation and furthermore the modification decrease its rate of degradition. – However P53 is controlled by a negative feedback loop so eventually the levels go back to their original low levels ([1] p 327) • Insulin is an example of a molecule that undergoes posttranslational modification. When it is translated it is in an inactive form and modification is required to active it. Exam question a) b) c) Prokaryotic DNA has a number of differences from Eukaryotic DNA Describe three differences between each type of DNA (9 marks) Explain how two of these differences result in extra steps in the Eukaryotic gene regulation process (12 marks) Explain, using a suitable example, how one of the extra regulatory steps will affect Eukaryotic DNA sequence analysis. (9 marks) • “Alternative splicing is a critical reason as to why the genome of humans is much smaller than would be expected”. 1. 2. 3. Explain how Alternative splicing ensures that the human geneome does not have to be as large as might be expected. (5 marks) Describe, using examples, 3 types of alternative splicing (15 marks) Discuss the potential impact alternative splicing may have on the analysis of Eukaryotic DNA coding sequences (10 marks). References • [1] Klug Essentials of Genetics: chapter 15 7th Edition • [2] Licatalosi, D.D. and Darnell, R.B. 2006. Splicing Regulation in Neurologic Disease. Neuron 52, 93-101 References • [3] http://www.ncbi.nlm.nih.gov/Class/MLACourse/ Modules/MolBioReview/alternative_splicing.html • [4] Modrek, B. and Lee, C. 2002. A genomic view of alternative splicing. Nature genetics 30, 13-19. • [5] http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1 370565/ • [6] IntronsIntron Retention Retention