3.11 & 4.2 Introduction to Reaction Stoichiometry
advertisement
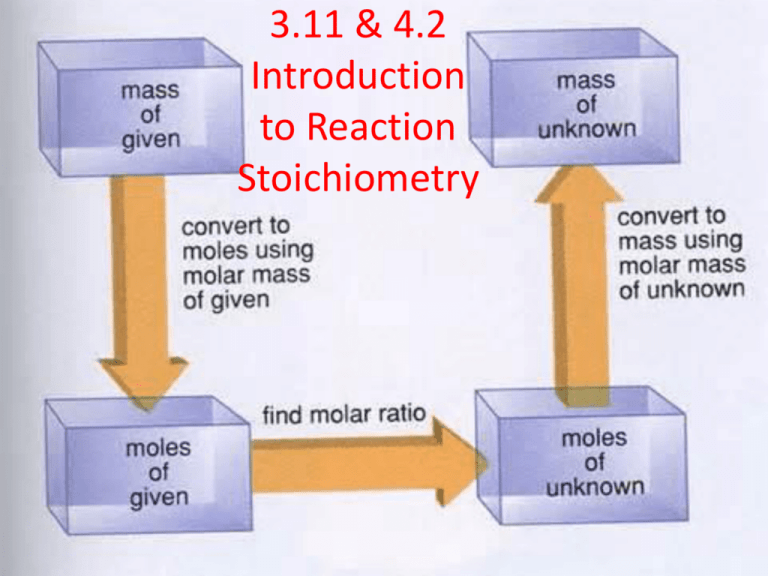
3.11 & 4.2 Introduction to Reaction Stoichiometry Writing and Balancing Chemical Equations How do you know if a chemical reaction has occurred? One or more substances have to have been converted into different substances. Let’s write the unbalanced chemical equation for the combustion on propane: C3H8(g) + O2(g) CO2(g) + H20(g) Does this equation obey the law of conservation of mass? How do you know? No, this equation does not obey the law of mass conservation because there is a different number of atoms, of each element, on both the reactant side and the product side of the chemical equation. How to Balance a Chemical Equation • Since atoms don’t just vanish or appear, we must balance the equation by listing coefficient which represents the mole ratio of each of the substances in the equation. Let’s try to balance the reaction on the previous slide: C3H8(g) + O2(g) CO2(g) + H20(g) C3H8(g) + 5O2(g) 3CO2(g) + 4H20(g) Let’s Try a Sample Problem Write the balanced equation for the reaction between aqueous lead(II) nitrate and aqueous potassium chloride to form solid lead(II) chloride and aqueous potassium nitrate. First let’s write the unbalanced equation: Pb(NO3)2(aq) + KCl(aq) PbCl2(s) + KNO3(aq) Now let’s balance it! Pb(NO3)2(aq) + 2KCl(aq) PbCl2(s) + 2KNO3(aq) Reaction Stoichiometry: How Much CO2? Balance the combustion of octane: C8H18(l) + O2(g) CO2(g) + H2O(g) 2C8H18(l) + 25O2(g) 16CO2(g) + 18H2O(g) Reaction stoichiometry is the numerical relationship between chemical amounts in a balanced chemical equation, and make predictions based on these amounts. A Chemical Recipe • We can compare reaction stoichiometry to a recipe. For example: (and we’ll keep it simple) Mr. Stevenson’s egg sandwich: 2 eggs + 4 bacon strips + 1 slice of cheese + 1 English muffin + butter Mr. Stevenson’s egg sandwich How many sandwiches could you make if you had 24 strips of bacon? 1 sandwich 24 strips of bacon X ------------------------- = 6 sandwiches 4 strips of bacon Mole-to-Mole Conversions Let’s use our balanced chemical equation for the combustion of octane to determine how many moles of CO2 enter our atmosphere when we combust 36.0 moles of octane. 2C8H18(l) + 25O2(g) 16CO2(g) + 18H2O(g) Step 1: List mol-to-mol ratio of octane to carbon dioxide. 2 mol octane: 16 mol carbon dioxide (1:8 ratio) Step 2: Use this ratio to covert moles of reactant to moles of product. 16 mol CO2 36 mol C8H18 X --------------------- = 288 mol CO2 2 mol C8H18 Mass-to-Mass Conversion Step 1: Convert the mass of what’s given to grams, if it’s not already in grams. Step 2: Convert grams of given to moles of given Step 3: Convert moles of given to moles of what you want. (Use mole-to-mole ratio) Step 3: Convert moles of what you want to mass in the units asked to find. Let’s Try a Sample Problem A component of acid rain is nitric acid, which forms NO2, also a pollutant, reacts with oxygen and water according to the simplified equation: 4NO2(g) + O2(g) + 2H2O(l) 4HNO3(aq) The generation of the electricity used by a n medium sized home produces about 16 kg of NO2 per year. Assuming that there is aqequate O2 and H2O, what mass of HNO3, in kg, can form from this amount of NO2 pollutant. Sample Problem 2 Worked Out I am going to complete this whole problem in one step using dimensional analysis. 1000 g NO2 1 mol NO2 4 mol HNO3 16 kg NO2 X ---------------- X ----------------- X ---------------1 kg NO2 46.01 g NO2 4 mol NO2 63.02 g HNO3 1 kg HNO3 ----------------- X -------------------- = 21.9 kg HNO3(aq) 1 mol HNO3 1000 g HNO3 Chapter 4 pg. 186 #’s 26, 28, 32, 36 (Just the a’s) Read 4.3 pgs. 145-151



