Necessary Nomenclature Knowledge
advertisement

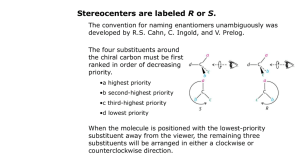
Necessary Nomenclature Knowledge The rules for naming molecules (IUPAC nomenclature) is complex, however, not every rule must be understood in order to successfully name the relatively smaller molecules that commonly occur in 14C. This guide provides a concise guide and summary to name those structures we worked so hard to assemble. (There have been past exam questions asking names) Main Idea It is necessary to understand that names consist of 5 parts, assembled using this table from right to left (i.e. functional group first, stereoisomerism last) Stereoisomerism Substituents Parent Unsaturation Functional group 1. Stereoisomerism Indicates whether double bonds are cis/trans, E/Z, and indicates stereocenters (R,S) 2. Substituents Groups coming off the main chains 3. Parent The main chain 4. Unsaturation Identifies if there are any double or triple bonds 5. Functional group The group after which the compound is named Quick Steps to remember (Start at the end of the name, working backwards) 1. 2. 3. 4. 5. Identify functional groups and order by hierarchy to determine suffix/prefix. Look for number and location of double and triple bonds. Identify the longest carbon chain as the parent chain. Label each substituents that are attached to the parent chain. Add Z/E (cis/trans) in the case of any present stereocenters or double bonds, (+/-) cannot be determined without a laboratory experiment Further explanation Functional Groups Functional groups are groups of atoms whose bonding is the same from molecule to molecule. These specific arrangements of atoms have certain characteristics for reactivity, i.e. molecules with identical functional groups have similar chemical and physical properties • • • If a compound has a functional group, add the indicated suffix to the name If a compound has more than one functional group, use the suffix of the functional group with greatest hierarchy and add the other functional group as a prefix in the substituent part of the name o Prefixed substituents are ordered alphabetically o If there are multiples functional groups of the same type, either prefixed or suffixed, the position numbers are ordered numerically If there is no functional group, end the name with an “e”. Priority 1 2 3 Functional Group Cations Carboxylic acids Carboxylic acid derivativesEsters Acyl halides Amides Imides Amidines Nitriles Aldehydes Ketones Alcohols Amines Ethers Peroxides 4 5 6 7 8 9 10 Formula NH4+ -COOH Prefix -oniocarboxy- Suffix -onium -oic acid -COOR -COX -CONH2 -CON=C< -C(=NH)NH2 -CN -CHO =O -OH -NH2 -O-OO- R-oxycarbonyl halocarbonylcarbamoyl-imidoamidinocyanoformyloxohydroxyamino-oxy-peroxy- -R-oate -oyl halide -amide -imide -amidine -nitrile -al -one -ol -amine Unsaturation Compounds with double or triple bonds are considered “unsaturated” because these compounds have less hydrogens. • • • • • # of Carbons Prefix • • The presence of a double or triple bonds is indicated with an “en” (double bond) and “yne” (triple bond). In the absence of a double or triple bond, use “an,” such as in the case of alkanes. If there are more than one double or triple bonds in a compound, add prefixes. 2 – di 3 - tri 4 - tetra 5 - penta 6 - hexa 7 - hepta 8 - octa 9 - nona 10 – deca If the compound has both double and triple bonds, list the double bond and then the triple bond. Parent Straight chain alkanes take the suffix “-ane” and are prefixed depending on the number of carbon atoms in the chain If there is a functional group, double bond, or triple bond, (in hierarchical order) include it in the chain even if this means a shorter carbon chain. o In the case of several functional groups, refer to functional group hierarchy as in the table above. 1 2 3 4 5 6 7 8 9 10 11 12 15 20 Meth Eth Prop But Pent Hex Hept Oct Non Dec Undec Dodec Pentadec Eicos It is of key importance to number the carbons in the parent chain. A similar hierarchy is used as when determining the parent chain itself. If present, the lowest number must be attributed to 1st. Functional Group a. The number gets placed directly in front of the suffix. 2nd. Double bond a. The number indicates the lower number of the two carbon atoms. 3rd. Triple bond a. Same as with double bond. 4th. Substituent (if more than one, label so that substituents get the lowest numbers possible) a. The number of the substituent goes immediately in front of the substituent. b. Every substituent must be numbered and if more than 1 are present, they are arranged in alphabetical order. i. Two numbers are separated by commas while letters and numbers are separated by dashes. Substituents Any atoms or groups, other than hydrogen, connected to the parent chain and hierarchical functional group (if present) are substituents. • Alkyl groups are named according to the carbon naming table presented immediately above, together with the “yl” ending. (i.e. methyl, ethyl, propyl) o If the substituent is not attached to the parent chain by its first carbon, but rather a middle carbon for example, it is a branched substituent. Common examples include: isopropyl and tert-butyl • • • Halogens are named as substituents by adding the letter “o” at the end. If the carbons in the parent chain are arranged in a ring, the term “cyclo” must be added to the beginning of the parent name. Every substituent needs to be numbered so that its position on the parent chain is clear. Stereoisomerism Stereoisomerism identifies the configuration of any double bonds or stereocenters. Remember that a stereoisomer is one molecule in a set of isomers that differ by the position of atoms in space, but are not constitutional isomers or conformational isomers. Stereoisomers are either enantiomers or diastereomers. • • If double bonds are present, they can be arranged in two ways, cis or trans. (Remember that double bonds cannot freely rotate at room temperature because of overlapping p orbitals) o If there are identical groups on the same side of a double bond, it is labeled as cis, if these identical groups are on other sides it is trans. Note- two identical groups attached to the same atom can only have one configuration and does not need this naming scheme. o If all four groups attached to the double bond are different there it is necessary to use Z/E naming, Z (meaning together) and E ( meaning opposite) The priority groups on the two sides of the double bond are compared, if on the same side then Z, if they are on the opposite side then E A molecule may have a region (stereocenter) where its atoms can be arranged in two different configurations, i.e. atoms can be left-handed (S) or right-handed (R). • o If more than one stereocenter is present, numbers must be used to denote the location of each stereocenter. o All attached groups to the stereocenter must be different, thus R/S would never be used to classify a double bond. o R- means the priority groups are attached in a clockwise fashion o S- means the priority groups are attached in a counterclockwise fashion. (+/-) is the direction in which a compound can rotate plane polarized light and is impossible to predict without a laboratory experiment. This has nothing to do with (R/S) and is a completely unrelated concept. Enantiomers rotate plane polarized light in opposite directions, however, it is impossible to make a prediction about which direction an unknown compound will rotate plane polarized light. o + means clockwise rotation o – means counterclockwise rotation (+/-) in the beginning of a name implies a racemic mixture (both enantiomers are present in the solution and the rotations cancel each other out. Sources Key concepts are taken from the recommended organic chemistry supplement: Klein, David R. Organic Chemistry as a Second Language: First Semester Topics. Hoboken, NJ: Wiley, 2012. Print. Definitions are from the illustrated glossary by Prof. Steve Hardinger: Hardinger, Steve. "Dr. Hardinger's Organic Chemistry Page - UCLA." Dr. Hardinger's Organic Chemistry Page - UCLA. UCLA Chemistry, n.d. Web. 08 June 2012. <http://www.chem.ucla.edu/harding/index.html>. Images are from wikipedia