apl_graphene_sup_rev4.11
advertisement

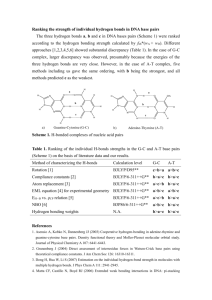
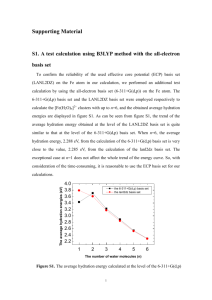
Supplementary Materials: Electron Spin Magnetism of Zigzag Graphene Nanoribbon Edge States Kun Xu* and Peide Ye School of Electrical and Computer Engineering and Birck Nanotechnology Center, Purdue University, West Lafayette, Indiana 47907 1. Theoretical methods The geometric parameters of the stationary points on the oxidation reaction potential-energy surfaces of the ZGNR(zigzag graphene nanoribbon) edge with O2/O3 were optimized by the Gaussian 09 program1 utilizing the B3LYP functional2,3 with 6-311G(d,p) and 3-21G(d) basis sets. For solid-gas reaction systems, the important part concerning atoms directly involved in the reaction (High level) was calculated by the 6-311G(d,p) basis set and the other atoms (Low level) by the 3-21G(d) basis sets, which was performed by the ONIOM (Our own N-layered Integrated molecular Orbital and molecular Mechanics) method4 implemented in Gaussian. The vibrational frequencies were calculated at every stationary point to determine the minimum energy structures and transition states. There is only one imaginary vibrational frequency at each transition state. In order to compare the singlet electronic state of ZGNR edge carbon radicals with its triplet state counterparts, more accurate energy calculations were performed by CASSCF5 (Complete Active Space Multi configuration SCF) and CIPT26 (a new method coupling multi reference configuration interaction and CASPT2) installed in the MOLPRO program.7 References 1 M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, J.A. Montgomery, Jr., T. Vreven, K.N. Kudin, J.C. Burant, J.M. Millam, S.S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G.A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J.E. Knox, H.P. Hratchian, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, P.Y. Ayala, K. Morokuma, G.A. Voth, P. Salvador, J.J. Dannenberg, V.G. Zakrzewski, S. Dapprich, A.D. Daniels, M.C. Strain, O. Farkas, D.K. Malick, A.D. Rabuck, K. Raghavachari, J.B. Foresman, J.V. Ortiz, Q. Cui, A.G. Baboul, S. Clifford, J. Cioslowski, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, M. Challacombe, P.M.W. Gill, B. Johnson, W. Chen, M.W. Wong, C. Gonzalez, and J.A. Pople, Gaussian 03, Revision C.02 (Gaussian, 2004). 2 A.D. Becke, J. Chem. Phys. 98, 5648 (1993). 3 Lee, Yang, and Parr, Phys. Rev. B Condens. Matter 37, 785 (1988). 4 S. Dapprich, I. Komáromi, K.S. Byun, K. Morokuma, and M.J. Frisch, J. Mol. Struct. THEOCHEM 461–462, 1 (1999). 5 P.J. Knowles and H.-J. Werner, Chem Phys Lett. 115, 259 (1985). 6 P. Celani, H. Stoll, H.-J. Werner, and P.J. Knowles *, Mol. Phys. 102, 2369 (2004). 7 H.-J. Werner, P.J. Knowles, G. Knizia, F.R. Manby, M. Schütz, and others, MOLPRO, Version 2009.1, a Package of Ab Initio Programs (Molpro, 2012). 2. Table SI. Comparison of energies of radical model systems calculated with CAS, CIPT2, and UB3LYP. Es and Et represent the energies of singlet and triplet radicals, respectively, in unit of hartree and Est(= Et- Es) in unit of eV. radical method Es/hartree Et/hartree Est/eV C10H6 CAS(8,10)/6-311G(d,p) -382.194698 -382.195737 -0.0283 H CIPT2(8,10)/6-311G(d,p) -383.443192 -383.443806 -0.0167 H UB3LYP/6-311G(d,p) -384.605581 -384.605229 0.0096 CAS(10,10)/6-31G(d) -686.088136 -686.088958 -0.0224 H CIPT2(8,10)/6-311G(d,p) -383.443192 -383.443806 -0.0247 H UB3LYP/6-311G(d,p) -690.5622338 -690.5622816 -0.0015 UB3LYP/6-311G(d,p) -1074.360543 -1074.361331 -0.0214 UB3LYP/6-311G(d,p) -1072.978973 -1072.979269 -0.0081 H H H H C18H8 H H H H H H C28H12 H H H H H H H H H H H H C28H10 H H H H H H H H H H 3. Table SII. Frontier occupied orbitals of C28H10 (1A) at the isovalue surface of 0.01and its orbital energies calculated at the UB3LYP level. Orbital -0.18692au -0.18619au -0.23020au -0.22887au -0.24680au -0.24699au HOMO HOMO1 HOMO2 HOMO3 -0.25414au -0.25852au HOMO4 -0.25962au -0.26075au HOMO5 -0.26726au -0.26690au HOMO6 -0.27427au -0.27103au 4. Figure S1. Adsorption reactions diagram for both O2(3Σ-g) with C10H6 and C28H12. O H H H H + H O2 (3-g) O H H H H + H H H C10H6O2 (1A') 3 C10H6 ( A') O H H H H + H O2 (3-g) O H H H H + H H H O O H H + H H H 1.8511eV (B3LYP) 1.9744eV (M06-2x) C10H6O2 (3A") C10H6 (1A') 1 2 4.7442 eV (B3LYP) 5.0576 eV (M06-2x) 5.3069 eV (MP2) H C10H6 (1A') O2 (3-g) 1 2 O O H H H H + H H C10H6O4 (1A) 1.7927 eV (B3LYP) 2.0073 eV (M06-2x) 1.9603 eV (MP2) H H H H + O2 (3-g) H H H H H H H H O H H H H H + H H O2 ( g ) H 3 - H H H H H H H O H H 4.6270 eV (B3LYP) 4.9064 eV (M06-2x) C28H12O2 (1A') H H + H H H C28H12 (3A') H H H H H O O H H + 1.9555 eV (B3LYP) 2.1105 eV (M06-2x) H H H H C28H12 (1A') H H H 3 C28H12O2 ( A") O O H H H H 1 2 H H H H H H 1 C28H12 ( A') H O H H H H O + O2 (3-g) 1 2 H H H H H H H H H C28H12O4 (1A) + 1.7944 eV (B3LYP) 2.0103 eV (M06-2x) 5. Table SIII. Data of partition functions, equilibrium constants, and desorption pressures for C28H12O2(3A”) → C28H12(1A’) + O2(3Σ-g) (∆𝐸 = 2.1106 eV) and C28H12O2(1A’) → C28H12(3A’) + O2(3Σ-g) (∆𝐸 = 4.6270 eV) at 293 K. C28H12O2(3A”) C28H12(1A’) O2(3Σ-g) qtrans 1.707×1032 qrot 70.2555 Kc(293K) PO2(Torr) qvib 172565 23830.2 1.00032 qelec 3 1 3 qtol 517695 23830.2 3.599×1034 8.188×10-4 14.96 C28H12O2(1A’) C28H12(3A’) O2(3Σ-g) Kc(293K) PO2(Torr) 1.046×10-45 1.911×10-41 qtrans 1.707×1032 qrot 70.2555 qvib 60125.4 22859.9 1.00032 qelec 1 3 3 qtol 60125.4 68579.7 3.599×1034 𝐾𝑐 = 𝑞𝐶28 𝐻12 𝑞𝑂2 𝑞𝐶28𝐻12 𝑂2 ∆𝐸 𝑒 −𝑅𝑇 [mol∙L-1] 𝑃𝑂2 = 𝑅𝑇𝐾𝑐 [Torr] R = 62.358 [Torr∙L∙K-1∙mol-1]