Balancing Equations
advertisement

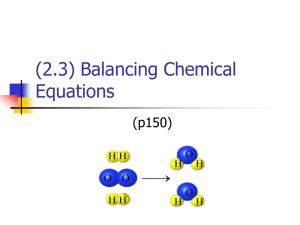
Balancing Equations CHEM Chemical equations tell you the following The substances that react together. The substances that are formed. The amounts of each substance involved. The arrow is read as "yields". HCl + NaOH NaCl + H2O The arrow always points from reactants to products Most chemical reactions are written with the arrow pointing from left to right like this . There are special situations that call for the arrow to be written pointing from right to left like this. Since the arrow always points from reactants to products, the reactants would be on the right if the arrow is pointing this way. There are chemical reactions that begin to form products and the products break down into the original reactants. These reactions are known as "reversible reactions" and are indicated with arrows pointing in both directions like this. Balanced Equations: The equation below is "balanced". S8 + 12O2 8SO3 Balanced equations have the same number of each type of atom on both sides of the arrow. Equations must be balanced because: Atoms can be neither created nor destroyed by ordinary chemical means, so there must be the same number of atoms on both sides of the equation. What law is this? Why do you think this law is so important to chemistry? These numbers are found in a chemical equation Subscripts The small numbers to the lower right of chemical symbols. Subscripts represent the number of atoms of each element in the molecule. Coefficients The large numbers in front of chemical formulas. Coefficients represent the number of molecules of the substance in the reaction. Just as subscripts of 1 are never written, coefficients of 1 are not written either. Both are "understood". The balanced equation S8 + 12O2 8SO3 tells us that one molecule of S8 reacts with twelve molecules of O2 to produce eight molecules of SO3. Using coefficients and subscripts to count atoms in equations: Multiply the coefficient in front of the chemical formula by the subscript after the atom. number of atoms = coefficient X subscript Example: How many atoms of hydrogen and oxygen are represented in 2H2O? •# of H atoms = coefficient 2 X subscript 2 = 4 •# of O atoms = coefficient 2 X subscript 1 = 2 Using coefficients and subscripts to count atoms in equations cont: Atoms found inside parenthesis in a formula have two subscripts. The subscript to the right of the parenthesis goes to all atoms inside. # of atoms = coefficient X subscript inside ( ) X subscript outside ( ) Example: How many of each type of atom are represented by: 2Al2(SO4)3 # of Al atoms = 2 X 2 = 4 # of S atoms = 2 X 1 X 3 = 6 # of O atoms = 2 X 4 X 3 = 24 7 steps to balance equations by inspection The order in which these steps are performed is important. While there are some shortcuts that can be used, following these steps in the order given below is the best way to be sure your equation is correct. 1. Check for Diatomic Molecules - H2 - N2 O2 - F2 - Cl2 - Br2 - I2 If these elements appear by themselves in an equation, they must be written with a subscript of 2 Balance equations by changing coefficients, never by changing subscripts in formulas. 2. Balance Metals 3. Balance Nonmetals 4. Balance Oxygen 5. Balance Hydrogen 6. Recount All Atoms If the atoms are not balanced at this point, there is a problem somewhere. Work your way back up the steps until you find the problem, and correct it. 7. If every coefficient will reduce, rewrite in the simplest whole-number ratio. An equation is not properly balanced if it is not written in its lowest whole-number ratio. Use the 7 steps to write the balanced equation for each of the following: NaOH Na2O + H2O Fe + O Fe2O3 CO2 + H2O C6H12O6 + O2 FeS + HCl FeCl2 + H2S O + H H2O Cl + NaI NaCl + I Al(NO3)3 + H2SO4 Al2(SO4)3 + HNO3 Check your Answers: 2 NaOH Na2O + H2O 4 Fe + 3O2 2 Fe2O3 6 CO2 + 6 H2O C6H12O6 + 6 O2 FeS + 2 HCl FeCl2 + H2S O2 + 2 H2 2 H2O Cl2 + 2 NaI 2 NaCl + I2 2 Al(NO3)3 + 3 H2SO4 Al2(SO4)3 + 6 HNO3