File
advertisement

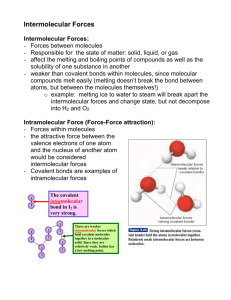
The physical properties of molecular substances result from different types of forces between their molecules. Intermolecular forces Temporary/instantaneous dipole Induced dipole London (dispersion) forces Permanent dipole Dipole-dipole interaction Van der Waal’s forces Hydrogen bonding INTERmolecular forces: forces between molecules Forces where whole molecules interact with each other INTRAmolecular forces: forces within molecules Forces that hold a single molecule together So what are examples of intramolecular forces? Examples of intermolecular forces? Just as the strength of the intramolecular forces can determine properties such as melting and boiling points, so can intermolecular forces The increase in intermolecular forces will Increase m.p. and b.p. (less volatile) Can determine the Solubility (like dissolves like) Conductivity (moving charges = conductivity) Non-polar molecules can have: Weak dipole = temporary/instantaneous dipole This can influence a neighbor molecule = induced dipole LDF strength increases as molecular size increases More e- in atom = more chances for temporary dipoles occurring LDF are ONLY forces between non-polar molecules But exist in all molecules!!! Generally have low m.p. and b.p. = Easy to break weak LDFs Polar covalent molecules have a permanent dipole These permanent charges orient other molecules the same way = dipole-dipole attraction Strength of the attraction depends on distance & orientation of dipoles similar molecular mass Note: A polar molecule can induce a dipole in a non- polar molecule = dipole-induced dipole Van der Waal forces: a term that includes both London dispersion forces and the dipole interactions (both dipole-dipole and dipole-induced dipole) Note: this term does not include H-bonding! Is a special case of dipole-dipole attraction Hydrogen bonded to: Nitrogen Fluorine Oxygen These are the strongest form of intermolecular attraction (so b.p. and m.p. are much higher than predicted) Can you explain why water is less dense in solid than in liquid form? H-bonds are the strongest intermolecular force, but is still weaker than covalent and ionic bonds What happens when CH3CH2OH is boiled? H2O and CO2 are released as gases CH3CH2OH is released as gas CH3CH3 and O2 are released as gases H2 and CO2 are released as gases So are intermolecular forces broken or intramolecular forces broken when something is boiled? Covalent cmpds have lower values than ionic Weak intermolecular forces vs electrostatic attraction Higher intermolecular bond strength, polar strength, and increase in molecular size increases m.p. and b.p. “Like dissolves like” Non-polar dissolve in non-polar (such as oil) Due to the formation of LDF Polar covalent molecules are usually soluble in water and other polar solvents Dipole interactions and H-bonding The larger the molecule with non-polar areas, the less polar the overall molecule Giant covalent are insoluble in nearly all solvents Too much E needed to break the bonds for interaction Covalent compounds do not conduct as there are no charges particles to carry a charge However, some polar covalent will conduct when in aqueous solution Giant covalent molecules Diamond = no conduction Fullerene & silicon = semiconductors Graphite & graphene = conductors Metallic bonds involve a lattice of cations with delocalized electrons. Metals have: Few valence electrons (typically) Low ionization energy Form positive ions by losing electrons when reacted When in elemental state, who can accept the e- s? These electrons tend to “wander off” or be delocalized These e- are no longer associated with 1 atom! The strength is determined by: Number of delocalized e- (more e-, stronger bond) Charge on cation (higher charge, stronger bond) Radius of the cation (smaller radius, stronger bond) Write the full e- configuration for Na and Mg: Compare the above points and determine which will have stronger bonding. Why? Melting point of Na = 98 °C Melting point of Mg = 650 °C Which atom will have a higher melting point: Na, K or Rb? How did you determine this? Melting point of Na = 98 °C Melting point of K = 63 °C Melting point of Rb = 39 °C Transition metals: very strong metallic bonds. Why? Many delocalized e- from 3d and 4s sub-shells Alloys: Solutions of metals Enhanced properties Typically solid but made when molten Metallic bonds can accommodate other cations of different sizes into lattice Bound by the delocalized e- in metal (thus metallically bonded) Have different properties than original components More chemically stable Often stronger Often more resistant to corrosion