Clinical Trial Required Documents Checklist
advertisement
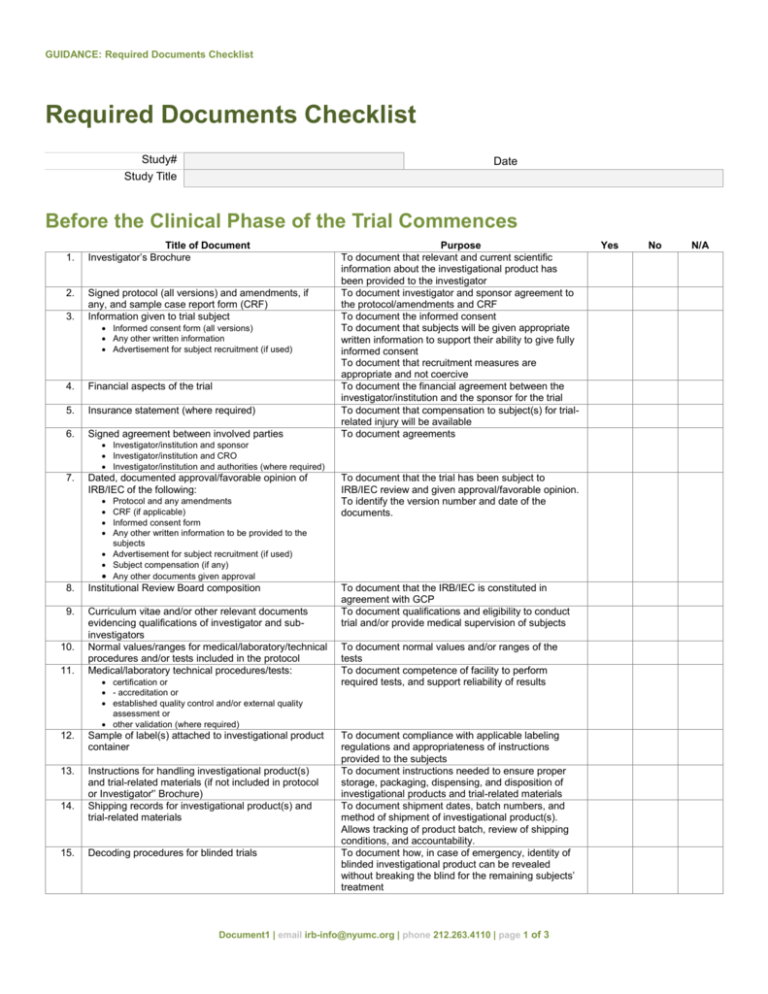
GUIDANCE: Required Documents Checklist Required Documents Checklist Study# Study Title Date Before the Clinical Phase of the Trial Commences 1. 2. 3. Title of Document Investigator’s Brochure Signed protocol (all versions) and amendments, if any, and sample case report form (CRF) Information given to trial subject Informed consent form (all versions) Any other written information Advertisement for subject recruitment (if used) 4. Financial aspects of the trial 5. Insurance statement (where required) 6. Signed agreement between involved parties Purpose To document that relevant and current scientific information about the investigational product has been provided to the investigator To document investigator and sponsor agreement to the protocol/amendments and CRF To document the informed consent To document that subjects will be given appropriate written information to support their ability to give fully informed consent To document that recruitment measures are appropriate and not coercive To document the financial agreement between the investigator/institution and the sponsor for the trial To document that compensation to subject(s) for trialrelated injury will be available To document agreements Investigator/institution and sponsor Investigator/institution and CRO Investigator/institution and authorities (where required) 7. Dated, documented approval/favorable opinion of IRB/IEC of the following: Protocol and any amendments CRF (if applicable) Informed consent form Any other written information to be provided to the subjects Advertisement for subject recruitment (if used) Subject compensation (if any) Any other documents given approval 8. Institutional Review Board composition 9. Curriculum vitae and/or other relevant documents evidencing qualifications of investigator and subinvestigators Normal values/ranges for medical/laboratory/technical procedures and/or tests included in the protocol Medical/laboratory technical procedures/tests: 10. 11. certification or - accreditation or established quality control and/or external quality assessment or other validation (where required) 12. Sample of label(s) attached to investigational product container 13. Instructions for handling investigational product(s) and trial-related materials (if not included in protocol or Investigator'’ Brochure) Shipping records for investigational product(s) and trial-related materials 14. 15. Decoding procedures for blinded trials To document that the trial has been subject to IRB/IEC review and given approval/favorable opinion. To identify the version number and date of the documents. To document that the IRB/IEC is constituted in agreement with GCP To document qualifications and eligibility to conduct trial and/or provide medical supervision of subjects To document normal values and/or ranges of the tests To document competence of facility to perform required tests, and support reliability of results To document compliance with applicable labeling regulations and appropriateness of instructions provided to the subjects To document instructions needed to ensure proper storage, packaging, dispensing, and disposition of investigational products and trial-related materials To document shipment dates, batch numbers, and method of shipment of investigational product(s). Allows tracking of product batch, review of shipping conditions, and accountability. To document how, in case of emergency, identity of blinded investigational product can be revealed without breaking the blind for the remaining subjects’ treatment Document1 | email irb-info@nyumc.org | phone 212.263.4110 | page 1 of 3 Yes No N/A GUIDANCE: Required Documents Checklist 16. Trial initiation monitoring report To document that trial procedures were reviewed with the investigator and investigator’s trial staff During the Clinical Conduct of the Trial 1. 2. 3. Title of Document Investigator’s Brochure updates Any revisions to: Protocol/amendments and CRF Informed consent form Any other written information provided to subjects Advertisement for subject recruitment (if used) Dated, documented approval/favorable opinion of IRB of the following: Protocol amendments Revisions of: Informed consent form Any other written information to be provided to the subject Advertisement for subject recruitment Any other documents given approval/favorable opinion Continuing review of trial 4. 5. 6. Curriculum vitae for new investigator(s) and/or subinvestigators Updates to normal values/ranges for medical laboratory/technical procedures/tests included in the protocol Updates of medical/laboratory technical procedures/tests: Purpose To document that investigator is informed in a timely manner of relevant information as it becomes available To document revisions of these trial-related documents that take effect during the trial To document that the amendment(s) and/or revision(s) have been subject to IRB review and were given approval/favorable opinion. To identify version number and date of the document(s). To document qualifications and eligibility to conduct trial and/or provide medical supervision of subjects To document normal values and ranges that are revised during the trial To document that tests remain adequate throughout the trial period certification or - accreditation or established quality control and/or external quality assessment or other validation (where required) 7. Documentation of investigational product(s) and trialrelated materials shipment 8. Monitoring visit reports 9. Relevant communications other than site visits: Letters Meeting notes Notes of telephone calls 10. Signed informed consent forms 11. Source documents 12. Signed, dated, and completed case report forms (CRFs) 13. Documentation of CRF corrections 14. Notification by originating investigator to sponsor of serious adverse events and related reports 15. Notification by investigator, where applicable, to IRB of unexpected serious adverse drug reactions and of other safety information 16. Notification by sponsor to investigator of safety information Interim or annual reports to IRB 17. To document shipment dates, batch numbers, and method of shipment of investigational product(s). Allows tracking of product batch, review of shipping conditions, and accountability To document site visits by, and findings of, the monitor To document any agreements or significant discussions regarding trial administration, protocol violations, trial conduct, adverse event (AE) reporting To document that consent is obtained in accordance with GCP and protocol, and dated prior to participation of each subject in the trial. Also to document direct access permission. To document the existence of the subject and substantiate integrity of trial data collected. To include original documents related to the trial, to medical treatment, and history of subject. To document that the investigator or authorized member of the investigator’s staff confirms the observations recorded To document all changes/additions or corrections made to CRF after initial data were recorded Notification by originating investigator to sponsor of serious adverse events and related reports in accordance with Safety Reporting Notification by investigator to IRB of unexpected serious adverse drug reactions in accordance with Adverse Drug Reaction Reporting and Safety Reporting guidelines and of other safety information In accordance with Safety Information Interim or annual reports provided to IRB in accordance with Progress Reports Document1 | email irb-info@nyumc.org | phone 212.263.4110 | page 2 of 3 Yes No N/A GUIDANCE: Required Documents Checklist 18. Subject Screening log 19. Subject identification code list 20. Subject enrollment log 21. Investigational product accountability at the site 22. Signature sheet 23. Record of retained body fluids/tissue samples (if any) To document identification of subjects who entered pretrial screening To document that investigator/institution keeps a confidential list of names of all subjects allocated to trial numbers on enrolling in the trial. Allows investigator/institution to reveal identity of any subject. To document chronological enrollment of subjects by trial numbers To document that investigational product has been used according to the protocol To document signatures and initials of all persons authorized to make entries and/or corrections on CRFs To document location and identification of retained samples if assays need to be repeated After Completion or Termination of the Trial 1. 2. Title of Document Investigational product accountability at site 3 Documentation of investigational product destruction (if applicable) Completed subject identification code list 4. 5. Audit certificate Final trial close-out monitoring report 6. Treatment allocation and decoding documentation 7. 8. Final report by investigator/institution to IRB Clinical study report Purpose To document that the investigational product(s) have been used according to the protocol. To document the final accounting of investigational products received at the site, dispensed to subjects, returned by the subjects, and returned to sponsor To document destruction of unused investigational products by sponsor or at site To permit identification of all subjects enrolled in the trial in case follow-up is required. List should be kept in a confidential manner and for agreed upon time. To document that audit was performed To document that all activities required for trial closeout are completed and copies of essential documents are held in the appropriate files Returned to sponsor to document any decoding that may have occurred To document completion of the trial To document results and interpretation of trial Document1 | email irb-info@nyumc.org | phone 212.263.4110 | page 3 of 3 Yes No N/A







