Ventilatory Failure
advertisement

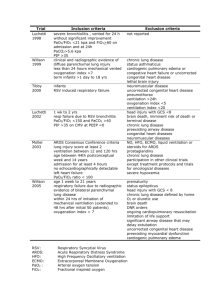
Respiratory Failure/ ARDS Ian B. Hoffman, MD, FCCP Pulmonary & Critical Care Medicine September 4, 2013 A 32-year-old man is evaluated for persistent hypoxemia on mechanical ventilation in the intensive care unit. His medical history is significant for paraplegia and a chronic indwelling urinary catheter for neurogenic bladder. He presented to the emergency department 2 days ago with sepsis. At that time, he received piperacillin/tazobactam, normal saline, and vasopressors. He was endotracheally intubated for decreased level of consciousness. His initial chest radiograph was normal. On physical examination on the second day of hospitalization, temperature is 37.1 °C (98.8 °F), blood pressure is 90/50 mm Hg, pulse rate is 96/min, and respiration rate is 26/min. His need for supplemental oxygen has steadily increased; his oxygen saturation on an FIO2 of 0.8 is 89%. Pulmonary examination reveals bilateral inspiratory crackles. Cardiac examination reveals distant, regular heart sounds. Urine and blood cultures are positive for Escherichia coli. A follow-up chest radiograph shows diffuse bilateral infiltrates without cardiomegaly. Central venous pressure is 8 mm Hg. Laboratory studies: Hemoglobin 13.2 g/dL (132 g/L) Leukocyte count 10,000/µL (10 × 109/L) Arterial blood gas studies (on an FIO2 of 0.8): pH 7.48 PCO2 30 mm Hg (4.0 kPa) PO2 60 mm Hg (8.0 kPa) Which of the following is the most likely cause of this patient’s hypoxemia? A. Acute respiratory distress syndrome B. E. coli pneumonia C. Heart failure D. Eosinophilic pneumonia Respiratory Failure Any disruption of function of respiratory system – CNS, nerves, muscles, pleura, lungs Any process resulting in low pO2 or high pCO2 – arbitrarily 50/50 Acute respiratory failure can be exacerbation of chronic disease or acute process in previously healthy lungs History 1940’s – polio, barbiturate OD 1960’s – blood gas analysis readily available, aware of hypoxemia 1970’s – decreased hypoxic mortality, increased multiorgan failure (living longer) 1973 – relationship between resp muscle fatigue and resp failure Types of Respiratory Failure Type 1 (nonventilatory) – hypoxemia with or without hypercapnia – disease involves lung itself (i.e, ARDS) Type 2 – failure of alveolar ventilation – decrease in minute ventilation or increase in dead space (i.e. COPD, drug OD) Goals of Treatment Correct hypoxemia or hypercapnia without causing additional complications Noninvasive ventilation vs. intubation and mechanical ventilation Goal of mechanical ventilation is NOT necessarily to normalize ABGs Ventilation–perfusion (V/Q) relationships and associated blood gas abnormalities Shunt The influence of shunt fraction on the relationship between the inspired oxygen (FiO2) and the arterial PO2 (PaO2). Ventilatory Failure Failure of respiratory pump to adequately eliminate CO2 pCO2 : VCO2 VA VCO2 determined by rate of total body metabolism ALVEOLAR HYPOVENTILATION IN THE ICU Respiratory Muscles Acute or acute-on-chronic overloading COPD, hyperinflation, fatigue Electrolyte imbalances Sepsis Shock Malnutrition Drugs Atrophy related to prolonged mechanical ventilation Hypothyroidism Myopathies What factors leading to respiratory muscle weakness can be reversed? Reduce respiratory load treat asthma, COPD, upper airway problems treat pneumonia, pulm edema, reduce dynamic hyperinflation, drain large pleural effusions, evacuate PTX Replace K, Mg, PO4, Ca Treat sepsis Nutritional support w/o overfeeding Rest muscles 24-48 hrs, then exercise Stop aminoglycosides Rule out hypothyroidism, oversedation, critical illness myopathy/neuropathy To intubate or not Decision to mechanically ventilate is clinical Some criteria: Decreased level of consciousness (ER always tells us that GCS = 3 and pt tubed to protect airway!) Vital capacity <15 ml/kg Severe hypoxemia Hypercarbia (acute or acute-on-chronic) Vd/Vt >0.60 NIF < -25 cm H20 ARDS – Acute Respiratory Distress Syndrome ARDS - Definition Severe end of the spectrum of acute lung injury Diffuse alveolar damage Acute and persistent lung inflammation with increased vascular permeability – inflammatory cytokines Diffuse infiltrates Hypoxemia No clinical evidence of elevated left atrial pressure (PCWP <18 if measured) ARDS – History/Definitions 1967 – Ashbaugh described 12 pts with acute respiratory distress, refractory cyanosis, decreased lung compliance, diffuse infiltrates; 7 of the 12 died 1988 – 4 point lung injury score (level of PEEP, pO2/FiO2, lung compliance, degree of infiltrates) 1994 – acute onset, bilateral infiltrates, no direct or clinical evidence of LV failure, pO2/FiO2) 1994 American European Consensus Acute Lung Injury ARDS Acute onset Acute onset Bilateral infiltrates c/w Bilateral infiltrates c/w pulmonary edema No clinical evidence of left-sided CHF (PCWP <18) pulmonary edema No clinical evidence of left-sided CHF (PCWP <18) paO2/FiO2 ratio <300 paO2/FiO2 ratio <200 100/0.40 = 250 100/0.60 = 167 New Definition of ARDS - 2012 • Acute onset (within 7 days of some defined event) • Bilateral infiltrates (on CXR or CT) • No need to exclude heart failure (respiratory failure “not fully explained by CHF”) • Hypoxemia – mild, moderate, severe Severity of ARDS (2012) ARDS Severity PaO2/FiO2 (on PEEP 5) Mortality Mild 200 - 300 27% Moderate 100 - 200 32% Severe <100 45% ARDS - Incidence Annual incidence 75 per 100,000 (1977) 9% of American critical care beds occupied by pts with ARDS ARDS - Diagnosis Clinically and radiographically resembles cardiogenic pulmonary edema PCWP can be misleading – should be normal or low, but can be high 20% of pts with ARDS may have LV dysfunction ARDS - Causes Direct injury to the lung Indirect injury to the lung in setting of systemic process Multiple predisposing disorders substantially increase risk Increased risk with alcohol abuse, chronic lung disease, acidemia ARDS - Causes Direct Lung Injury Pneumonia Gastric aspiration Lung contusion Fat emboli Near drowning Inhalation injury Reperfusion injury Indirect Lung Injury Sepsis Multiple trauma Cardiopulm bypass Drug overdose Acute pancreatitis Blood transfusion ARDS - Physiologic Derangements Inflammatory injury producing diffuse alveolar damage Alveolar epithelium (eg, aspiration) Vascular endothelium (eg, sepsis) Proinflammatory cytokines (TNF, IL-1, IL-8) Neutrophils recruited – release toxic mediators Normal barriers to alveolar edema are lost, protein and fluid flow into air spaces, surfactant lost, alveoli collapse; inhomogeneous process Impaired gas exchange Decreased compliance Pulmonary hypertension ARDS – Features Severe initial hypoxemia Increased work of breathing (decreased compliance) – generally a prolonged need for mechanical ventilation Initial exudative stage Proliferative stage resolution of edema, proliferation of type II pneumocytes, squamous metaplasia, collagen deposition Fibrotic stage ARDS – Course Early Inciting event pulmonary dysfunction (worsening tachypnea, dyspnea, refractory hypoxemia) Nonspecific labs CXR – diffuse alveolar infiltrates Subsequent Eventual improvement in oxygenation Continued ventilator dependence Complications Large dead space, high minute ventilation requirement Organization and fibrosis in proliferative phase ARDS - Complications Ventilator induced lung injury Sedation and neuromuscular blockade Nosocomial infection Pulmonary emboli Multiple organ dysfunction ARDS - Prognosis Improved survival in recent years – mortality was 50- 60% for many years, now 35-40% Improvements in supportive care, improved mechanical ventilatory management Early deaths (3 days) usually from underlying cause of ARDS Later deaths from nosocomial infections, sepsis, MOSF Respiratory failure only responsible for ~16% of fatalities Long-term survivors usually show mild abnormalities in pulmonary function (DLCO) Question 2 • A 63-year-old man with acute respiratory distress syndrome (ARDS) is evaluated in the intensive care unit. He has just been intubated and placed on mechanical ventilation for ARDS secondary to aspiration pneumonia. Before intubation, his oxygen saturation was 78% breathing 100% oxygen with a nonrebreather mask. • On physical examination, temperature is 37.0 °C (98.6 °F), blood pressure is 150/90 mm Hg, and pulse rate is 108/min. His height is 150 cm (59 in) and his weight is 70.0 kg (154.3 lb). Ideal body weight is calculated to be 52.0 kg (114.6 lb). Central venous pressure is 8 cm H2O. Cardiac examination reveals normal heart sounds and no murmurs. Crackles are auscultated in the lower left lung field. The patient is sedated. Neurologic examination is nonfocal. • Mechanical ventilation is on the assist/control mode at a rate of 18/min. Positive end-expiratory pressure is 8 cm H2O, and FIO2 is 1.0. Which of the following is the most appropriate tidal volume? A. 300 ml B. 450 ml C. 700 ml D. 840 ml Ventilatory Goals in ARDS Provide adequate oxygenation without causing damage related to: Oxygen toxicity Hemodynamic compromise Barotrauma Alveolar overdistension Alveolar shear Mechanical Ventilation in ARDS Reliable oxygen supplementation Decrease work of breathing Increased due to high ventilatory requirements, increased dead space, and decreased compliance Recruitment of atelectatic lung units Decreased venous return can help decrease fluid movement into alveolar spaces Ventilator Induced Lung Injury Known for decades that high levels of positive pressure ventilation can rupture alveolar units In 1950’s became known that high FiO2 can produce lung injury More recently, effects of alveolar overdistension, shearing, cyclical opening and closing have become apparent Ventilator Induced Lung Injury Macrobarotrauma Pneumothorax, interstitial emphysema, pneumomediastinum, SQ emphysema, pneumoperitoneum, air embolism ? resulting from high airway pressures, or just a marker of severe lung injury Higher PEEP predicts barotrauma Ventilator Induced Lung lnjury Microbarotrauma Alveolar overinflation exacerbating and perpetuating lung injury – edema, surfactant abnormalities, inflammation, hemorrhage Less affected lung accommodates most of tidal volume – regional overinflation Cyclical atelectasis (shear) – adds to injury Low tidal volume strategy (initial tidal volume 6 ml/kg IBW, plateau pressure <30) – lower mortality Ventilatory Strategies Therapeutic target of mechanical ventilation in patients with ARDS has shifted from maintenance of "normal gas exchange” to the protection of the lung from ventilator-induced lung injury Low tidal volume, plateau pressure <30 peak pressure = large airways plateau pressure = small airways/alveoli PEEP – enough, not too much Pressure controlled vs. volume cycled Prolonging inspiratory time (increase mean airway pressure and improve oxygenation) APRV Recent data suggests high frequency oscillation is bad Permissive hypercapnia Secondary effect of low tidal volumes Maintain adequate oxygenation with less risk of barotrauma Sedation/paralysis often necessary The only method of mechanical ventilation that has been shown in randomized controlled trials to improve survival in patients with ARDS is low tidal volume ventilation. ARDS Network Trial NEJM 2000; 342:1301-1308. Initial tidal volume of 6 ml/kg IBW and plateau pressure <30 vs. Initial tidal volume of 12 ml/kg IBW and plateau pressure <50 Reduction in mortality of 22% (31% vs 40%) Ventilator management in patients with acute respiratory distress syndrome or acute lung injury N Engl J Med 2000; 342:1301 Question 3 A 25-year-old woman is admitted to the intensive care unit (ICU) for a 6hour history of respiratory distress. She has acute lymphoblastic leukemia and received cytotoxic chemotherapy 2 weeks before ICU admission. She has had fever and leukopenia for 7 days. On physical examination, she is in marked respiratory distress. Temperature is 39.0 °C (102.2 °F), blood pressure is 110/70 mm Hg, pulse rate is 130/min, and respiration rate is 42/min. Weight is 50.0 kg (110.2 lb). Ideal body weight is calculated as 50.0 kg (110.2 lb). Acute respiratory distress syndrome is diagnosed. She is intubated and started on mechanical ventilation in the assist/control mode at a rate of 12/min, tidal volume of 300 mL, positive end-expiratory pressure (PEEP) of 5 cm H2O, and FIO2 of 1.0. An arterial blood gas study on these settings shows a pH of 7.47, PCO2 of 30 mm Hg (4.0 kPa), and PO2 of 45 mm Hg (6.0 kPa). Peak airway pressure is 26 cm H2O, and the plateau pressure is 24 cm H2O. Which of the following is the most appropriate treatment to improve this patient’s oxygenation? A. Increase PEEP to 10 cm H2O B. Increase respiratory rate to 18/min C. Increase tidal volume to 500 ml D. Start inhaled nitric oxide PEEP in ARDS Increases FRC (volume of air remaining in lungs following a normal tidal exhalation) – recruits “recruitable” alveoli, increases surface area for gas exchange Decreases shunt, improves V/Q matching No consensus on optimal level of PEEP ALVEOLI trial NEJM 2004; 351:327-336. High PEEP vs. low PEEP Low tidal volume for all (6 ml/kg predicted weight) Higher PEEP patients had better oxygenation, but no difference in mortality, duration of mechanical ventilation, duration of non-pulmonary organ failure No benefit from recruitment maneuvers (CPAP 35-40 cm H20 for 30 seconds) – but other studies suggest that recruitment maneuvers do help Prone Positioning Thought to improve oxygenation and respiratory mechanics by: alveolar recruitment redistribution of ventilation toward dorsal areas resulting in improved V/Q matching elimination of compression of the lungs by the heart reduction of parenchymal lung stress and strain Prone Positioning Several studies demonstrate improved oxygenation, but no overall reduction in mortality Greatest benefit of prone positioning occurs in the sickest patients if used early after the diagnosis of ARDS Other modalities - None of these have proven superior to more standard techniques APRV High-frequency ventilation Partial liquid ventilation Inverse ratio ventilation ECMO Nitric Oxide, prostacyclin Ketoconazole, ibuprofen Glutathione (anti-oxidant) Surfactant Steroids Intravenous beta-agonists (increases clearance of alveolar edema) – needs more study APRV Pharmacotherapy - Nitric Oxide Selectively dilates vessels that perfuse better ventilated lung zones, resulting in improved V/Q matching, improved oxygenation, reduction of pulmonary hypertension Less benefit in septic patients No clear improvement in mortality Pharmacotherapy - Surfactant First tried in 1980’s No benefit in adult population One study did demonstrate improvement in oxygenation and mortality in children Pharmacotherapy - Steroids No consensus on effectiveness – no clear benefit, some risks ARDSnet - NEJM 2006; 354:1671-1684. some benefit in subgroups, but not overall; increased mortality if started after 14 days; neuromyopathy Meduri - Chest 2007; 131:954-963. improvement in pulmonary and extrapulmonary organ dysfunction, reduction in duration of mechanical ventilation and ICU length of stay – (small sample size, imbalance in treatment arms) Fluid management in ARDS Increased extravascular lung water associated with poor outcome Reduction in PCWP associated with increased survival Fluid and Catheter Treatment Trial (FACTT) NEJM 2006 Liberal vs conservative fluid management CVP just as good as PCWP Conservative management group did better (more ventilator free days, fewer ICU days, trend toward lower mortality) No difference in incidence of hypotension or need for renal replacement therapy Excluded patients with shock, was initiated later in ICU course (mean time 43 hrs) – early aggressive fluid resuscitation appropriate Liberal group gained ~1 liter/day, conservative had net zero balance over 1st 7 days Simplified Algorithm for Conservative Fluid Management (Target CVP <4 or PCWP <8) MAP > 60, no vasopressors for > 12 hrs CVP PCWP Average urine output <0.5 cc/kg/hr Average urine output >0.5 cc/kg/hr >8 >12 Lasix; reassess in 1 hr Lasix; reassess in 4 hrs 4-8 8-12 Rapid fluid bolus; reassess 1 hr Lasix; reassess in 4 hrs <4 <8 Rapid fluid bolus; reassess 1 hr No intervention; reassess in 4 hrs Supportive Care Treat predisposing factors Prophylaxis for GI bleeding DVT prophylaxis Prevent and treat nosocomial pneumonia – most important causes are microaspiration, biofilm formation (VAP bundle?) Nutritional support Blood sugar control ?Transfusion (Hgb >7 adequate) Decrease oxygen utilization - antipyretics, sedatives, paralysis VAP Bundle – ?truly evidence based Elevate head of bed (helpful) Daily sedation vacation and assessment of readiness to extubate (shorter duration on vent, should be less pneumonia) Daily chlorhexidine mouth rinse (questionable benefit) PPI or H2 antagonists (can increase risk) DVT prophylaxis (nothing to do with pneumonia) Potentially helpful: subglottic suctioning, lateral headdown positioning, silver-coated ET tubes, “mucus shaver” “There is nothing so useless as doing efficiently that which should not be done at all.” (Peter Drucker) Question 4 A 50-year-old man is evaluated in the intensive care unit for acute respiratory distress syndrome secondary to severe community-acquired pneumonia. He is intubated and placed on mechanical ventilation. He was previously healthy and took no medications before his hospitalization. On physical examination, temperature is 38.3 °C (100.9 °F), blood pressure is 120/60 mm Hg, and pulse rate is 110/min. The patient weighs 60.0 kg (132.3 lb); ideal body weight is 60.0 kg (132.3 lb). He is sedated and is not using accessory muscles to breathe. Central venous pressure is 8 cm H2O. Other than tachycardia, cardiac examination is normal. There are bilateral inspiratory crackles. Initial ventilator settings are volume control with a rate of 18/min, a tidal volume of 360 mL, positive end-expiratory pressure (PEEP) of 10 cm H2O, an FIO2 of 0.8, a peak pressure of 34 cm H2O, and a plateau pressure of 32 cm H2O. Oxygen saturation by pulse oximetry is 96%. Which of the following is the most appropriate next step in management? A. Decrease respiratory rate B. Decrease tidal volume C. Increase FiO2 D. Increase PEEP Simplified Algorithm for Conservative Fluid Management Shock CVP No Shock Oliguric Non-Oliguric >9 Vasopressor Diuretic Diuretic 4-8 Fluid bolus Fluid bolus Diuretic <4 Fluid bolus Fluid bolus KVO fluid Flow diagram for the evaluation of hypoxemia PVO2 = mixed venous pO2 VO2 = oxygen consumption DO2 = oxygen delivery Flow diagram for the evaluation of hypercapnia VCO2 = CO2 production