Adapted from Carley Karsten Bio93 Discussion TA: Alberto Lopez e
advertisement

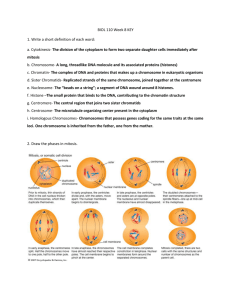
Adapted from Carley Karsten Bio93 Discussion TA: Alberto Lopez e-mail: ajlopez1@uci.edu Lecture 14-15 Study Guide I have organized some terms and topic that I think are important. This does not mean that other topics mentioned during lecture or in the book will not be tested. This guide is meant to clarify and emphasize certain points, NOT to list everything you need to know. I will focus on tying things together across lectures, and giving real-life examples of the biological principles that we are learning. Details that I include that I think will be helpful, but that you don’t need to know, I will write in green. Questions to think about I will write in blue. Lecture 14: Cell cycle regulation G1 checkpoint: checks if environmental conditions are ok for cell division pass: continue to S phase fail: exit cell cycle and goes to G0. This is a nondividing state. Most of the cells in your body right now are in G0. G2 checkpoint: checks that newly-synthesized DNA (from S phase) is not damaged M checkpoint: checks that all kinetochores are attached to mitotic spindles Important regulatory molecules: cyclin-dependent kinase (CDK): enzymes that promote cell division; “gas pedal”. ONLY works when bound to a cyclin. cyclin: accumulates during the cell cycle, and is degraded afterwards this is important. Remember that the amounts of CDK don’t change during the cell cycle. The only thing that changes is CDK activity, and this depends on how much cyclin is around. tumor suppressors: inhibit cell division; “brakes” *G1 and G2 checkpoints both use cyclin + CDK. The one we talked about most in class was for G2: the accumulation of a cyclin that allows MPF to be active. MPF is just another name for a particular combination of cyclin + CDK (cyclin B + CDK1, if you’re interested). What happens to cells that fail the G2 or M checkpoints? What do CDKs actually *do*? Start by figuring out what kinases in general do. Then figure out what’s special about CDKs. Cell cycle regulation and cancer Here I will describe some characteristics of normal cell cycle regulation, and how they can be messed up to lead to cancer. Remember that cancer, in simple terms, is uncontrolled cell division. 1. presence of growth factors a. normal cells only divide when there are growth factors (such as PDGF or BDNF) around b. cancer cells can get around this restriction by two possible methods… i. they make their own growth factor, so that they don’t need to rely on Adapted from Carley Karsten the body to provide it for them ii. they have mutated signaling pathways that are always on even if there aren’t growth factors around *example: tyrosine kinase receptors are growth factor receptors. In many types of cancer, tyrosine kinase receptors are mutated so that they are always stuck together (dimerized), and therefore always active. This tricks the cancer cell into thinking that there are always growth factors around. 2. anchorage dependence a. normal cells only divide when they are attached to a surface b. cancer cells can divide even if they’re unattached. This leads to problems like metastasis, where cancer cells break off from tumors and travel through the bloodstream to start new tumors elsewhere in the body. What type of membrane protein that we talked about earlier in this class might be mutated in cancer cells to mess up anchorage dependence? 3. density-dependent inhibition a. normal cells stop dividing when they feel crowded by other cells Why is this beneficial? Think about things like nutrients and waste… b. cancer cells don’t care. They form dense tumors, and keep growing anyway. 4. things we didn’t talk about in class, but are really cool if you’re interested… (if you’re REALLY interested, check out the paper I posted on the class website: “The Hallmarks of Cancer”) a. apoptosis i. normal cells are destroyed if their DNA gets too damaged. This process is called apoptosis. ii. cancer cells are often mutated in a way that allows them to escape apoptosis signals, and survive even with major damage. b. angiogenesis i. normal cells have to be really close to blood vessels in order to get all the nutrients they need. This is part of the reason they exhibit densitydependent inhibition. ii. cancer cells can sometimes grow their own blood vessels, allowing even super-dense tumors to get nutrients to all of their cells. c. limitless replication i. normal cells can only divide a set number of times (usually ~60 times). When they’re done, they’re done. This has a lot to do with telomeres, which we will be talking about later on in this course. When telomeres get too short, the cell won’t divide any more. ii. cancer cells are often “immortalized”, meaning that they don’t ever stop dividing. This often involves mutation of telomere-related enzymes, such that telomeres never get short. The book “The Immortal Life of Henrietta Lacks” is about a woman whose cancer cells were harvested for research decades ago, and are still being used in labs all around the world because they will divide forever. Adapted from Carley Karsten More on cancer: proto-oncogenes vs. oncogenes This can be confusing. The most important thing to remember is that proto-oncogenes do not cause cancer. However, they have the potential to cause cancer if they were to be mutated. When proto-oncogenes are mutated, they are called oncogenes. In general, protooncogenes are genes that promote cell division, and they turn into oncogenes when they are mutated in a way that makes them active all the time. Example: the gene for cyclin B (part of MPF cyclin/CDK complex) is a proto-oncogene. Why? Because if you mess it up, you could get cancer. If you mutate the gene for cyclin B so that it can’t be degraded by the proteasome, it is now called an oncogene. Why? Because it causes cancer. How does it cause cancer? Since it can’t be degraded, it is always present, and always bound to CDK1. When CDK1 is bound to cyclin B, it is active, and promotes cell division by allowing passage through the G2 checkpoint. Your turn. Looking at the characteristics of normal cells and cancer cells above, what are some other proto-oncogenes you could think of? How could these proto-oncogenes be mutated to become oncogenes? I’ll help get you started with an example (also described above): -- proto-oncogene: tyrosine kinase receptor -- to make it into an oncogene: mutate it so that the receptors are always dimerized, and therefore always active. This will trick the cell into thinking that there are always growth factors present, allowing the cell to divide even in unfavorable environments. Can tumor suppressor genes be considered proto-oncogenes? Why or why not? Lecture 15: Meiosis Terminology 1. sister chromatids: identical copies of a duplicated chromosome. Have the same alleles of the same genes. 2. nonsister chromatids: homologous pair. Have different alleles of the same genes. 3. homologous pair: 1 maternal chromosome + 1 paternal chromosome; different alleles of the same genes 4. diploid cell: contains the normal amount of genetic material (2 copies of each chromosome) Diploid human cells contain how many chromosomes? 5. haploid cell: contains half the normal amount of genetic material (as in gametes, which possess only one copy of each chromosome) 6. synapsis: the physical connection of homologous pairs of chromosomes. The “glue” that holds them together is called the synaptonemal complex. If the synaptonemal complex were disturbed, what would happen? (or… what would *not* happen?) Adapted from Carley Karsten 7. chiasmata: point between homologous pairs at which crossing over has occurred. Looks like an “X” between the chromosomes, because they’re sort of attached to each other. 8. nondisjunction: sister chromatids fail to separate during anaphase (see below) Which is worse: nondisjunction during Anaphase I or Anaphase II? Why? 9. allele: different version of a gene. For example, the gene for eye color could exist as three possible alleles: blue, green, and brown. We will talk more about this in the next lecture. Meiosis = duplication + division + division. Duplication occurs in interphase. 1st division occurs in MI. 2nd division occurs in MII. Interphase: diploid cell diploid cell with duplicated chromosomes just like interphase for mitosis, chromosomes replicate to form sister chromatids Meiosis I: diploid cell with duplicated chromosomes two haploid cells, each with duplicated chromosomes 1. Prophase I: crossing over occurs. This is super important: crossing over is one event that gives rise to genetic diversity!! Random chunks of each nonsister chromatid are exchanged. 2. Metaphase I: just like mitosis metaphase, but lining up homologous pairs rather than sister chromatids. a. nonrandom: pairing of homologous chromosomes. They always line up next to one another. b. random: polarity. For each homologous pair, the maternal or paternal chromosome could end up going either way. 3. Anaphase I: homologous pairs separate, but sister chromatids stay together. If nondisjunction occurs here, all of your daughter cells will have the wrong number of chromosomes 4. Telophase I and Cytokinesis: just like telophase / cytokinesis for mitosis, except that the two cells that we get at the end are each haploid (haploid meaning that they don’t have two sets of chromosomes, although they do have two copies of chromosomes- this is an important distinction. If you’re confused at all, draw it out or look at the diagrams in the book for a long time or come to office hours!) Meiosis II: 2 haploid cells, each with duplicated chromosomes 4 haploid cells 1. Prophase II: nothing unique here, just normal prophase stuff 2. Metaphase II: normal metaphase. Only difference here is that the sister chromatids are NOT IDENTICAL. Why not? (think about what happened during Meiosis I) 3. Anaphase II: normal If nondisjunction occurs here, only half of your daughter cells will have the wrong number of chromosomes. 4. Telophase II and Cytokinesis: normal. The four cells that we get at the end are each haploid (this time they’re haploid for real. Only one copy of each chromosome of a single set) Adapted from Carley Karsten Sources of genetic variability: THIS IS VERY IMPORTANT 1. Independent assortment of chromosomes during metaphase (I and II) 2. Crossing over during prophase I 3. Random fertilization Ignoring all the math and stuff, be able to describe how each of these factors contributes to genetic diversity This is the basis of life as we know it. Without these sources of variability, evolution would take SOOOO much longer. The only other sources of genetic variation are environmental effects like radiation… not so good. A common misconception about evolution is that environments “cause” genes to adapt- this is not true. What happens is that genes randomly combine and mutate due to things like crossing over, and then the combinations that work best in a given environment are the ones that survive longer and produce more children, thus increasing the chances of passing on those same genes.