Chapter 25 Water, Electrolyte, and Acid

Chapter 25
Water, Electrolyte, and Acid-Base Balance
Elsevier items and derived items © 2007, 2003, 2000 by Saunders, an imprint of Elsevier Inc.
Slide 1
Introduction
• Water, electrolytes, acids, and bases are tightly regulated. Fluids and electrolytes must be distributed in the body compartments in the correct volumes and concentrations.
Slide 2
Body Fluids:
Distribution and Composition
• Major Fluid Compartments
– The two major fluid compartments are the intracellular compartment (63% of the water) and the extracellular compartment (37% of the water).
– The extracellular compartment includes interstitial fluid, intravascular fluid (plasma), lymph, and transcellular fluid.
Slide 3
Body Fluids:
Distribution and Composition
- cont’d
• Composition of Body Fluids
– Intracellular fluid contains high concentrations of potassium (K + ), phosphate (PO magnesium (Mg 2+ ).
4
3– ), and
– Extracellular fluid contains a high concentration of sodium (Na + ), chloride (Cl – ), and bicarbonate
(HCO –
3
).
Slide 4
Water Balance: Intake Equals Output
• Intake
– The average intake of water is 2500 ml/24 hrs.
– The primary regulator of fluid intake is thirst.
• Output
– The average output of water is 2500 ml/24 hrs.
– Water is excreted by the kidneys (60%); skin and lungs
(28%); digestive tract (6%); and sweat (6%).
• Water: Deficiency and Excess
– A deficiency of body water is called dehydration.
– An excess of body water expands blood volume and causes edema.
Slide 5
Electrolyte Balance
• Sodium (Na + )
– The chief extracellular cation.
– Necessary for nerve-muscle conduction and to maintain fluid balance.
– Primarily regulated by aldosterone.
Slide 6
Electrolyte Balance
- cont’d
• Potassium (K + )
– The chief intracellular cation.
– Necessary for nerve-muscle conduction.
– Primarily regulated by aldosterone.
Slide 7
Electrolyte Balance
- cont’d
• Calcium (Ca 2+ )
– Strengthens bones and teeth and is necessary for muscle contraction, nerve-muscle conduction, and blood clotting.
– Is primarily regulated by parathyroid hormone.
Slide 8
Electrolyte Balance
- cont’d
• Magnesium (Mg 2+ ) and Chloride (Cl – )
– Magnesium performs important functions in heart, muscles, and nerves.
– Chloride is the chief extracellular anion and follows Na + .
Slide 9
Electrolyte Balance
- cont’d
• Bicarbonate (HCO –
3
)
– A major extracellular anion.
– Plays three important roles: it is the major form in which CO
2 is transported, it acts as a base
(alkaline substance), and it plays an important role in acid-base balance.
Slide 10
Acid-Base Balance
• pH
– The pH refers to the concentration of H + .
– The normal blood pH is 7.35 to 7.45. If blood pH is less than 7.35, the person is in acidosis. If blood pH is greater than 7.45, the person is in alkalosis.
Slide 11
Acid-Base Balance
- cont’d
• Regulation of Blood pH
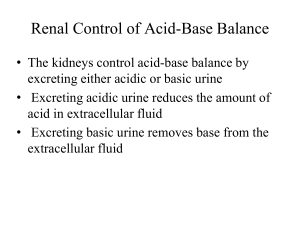
– Blood pH is regulated by three mechanisms: buffers, the respiratory system, and the kidneys.
– Buffers (the buffer pair) can donate or remove H + .
– The respiratory system affects pH by regulating
CO
2
.
– The kidneys can vary their excretion of H + .
Slide 12
Acid-Base Imbalances
• Acidosis
– Acidosis means that the blood pH is less than 7.35.
– Respiratory acidosis is caused by hypoventilation.
– Metabolic acidosis is due to nonrespiratory causes, including kidney disease, uncontrolled diabetes mellitus, diarrhea, and lactic acid production.
Slide 13
Acid-Base Imbalances
- cont’d
• Alkalosis
– Alkalosis means that the blood pH is greater than
7.45.
– Respiratory alkalosis is caused by hyperventilation.
– Metabolic alkalosis is caused by nonrespiratory conditions, including overingestion of antacids and loss of gastric contents.
Slide 14